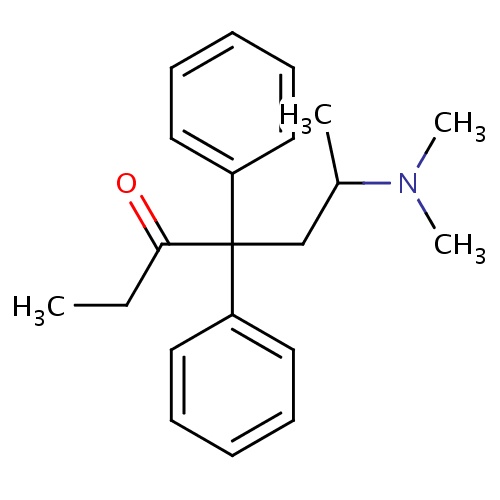
IUPAC name
6-(dimethylamino)-4,4-diphenylheptan-3-one
SMILES
CCC(=O)C(CC(C)N(C)C)(C1=CC=CC=C1)C1=CC=CC=C1
Compound class
Analgesics; Analgesics, Opioid; Narcotics; Antitussive Agents; Nervous System; Drugs Used in Addictive Disorders; Opioids; Diphenylpropylamine Derivatives; Drugs Used in Opioid Dependence; Cytochrome P-450 CYP1A2 Inhibitors; Cytochrome P-450 CYP2C9 Inhibitors; Cytochrome P-450 CYP1A2 Inducers; Cytochrome P-450 CYP2C8 Inhibitors; Cytochrome P-450 CYP2C9 Inducers; Cytochrome P-450 CYP2C19 Inducers; Cytochrome P-450 CYP2C8 Inducers; Cytochrome P-450 CYP2B6 Inducers; Cytochrome P-450 CYP2B6 Inhibitors; CYP2B6 Inhibitors (strong); CYP2D6 Inducers; CYP2D6 Inducers (strong); CYP3A4 Inhibitors; Antiemetics Antagonists; Combined Inhibitors of CYP3A4 and P-glycoprotein;
Therapeutic area
For the treatment of dry cough, drug withdrawal syndrome, opioid type drug dependence, and pain.
Common name
Methadone
IUPAC name
6-(dimethylamino)-4,4-diphenylheptan-3-one
SMILES
CCC(=O)C(CC(C)N(C)C)(C1=CC=CC=C1)C1=CC=CC=C1
INCHI
InChI=1S/C21H27NO/c1-5-20(23)21(16-17(2)22(3)4,18-12-8-6-9-13-18)19-14-10-7-11-15-19/h6-15,17H,5,16H2,1-4H3
FORMULA
C21H27NO

Common name
Methadone
IUPAC name
6-(dimethylamino)-4,4-diphenylheptan-3-one
Molecular weight
309.445
clogP
4.707
clogS
-5.126
HBond Acceptor
2
HBond Donor
0
Total Polar Surface Area
20.31
Number of Rings
2
Rotatable Bond
7
| Drug ID | Common name | Structure CAS | SMILE | Frequency |
|---|---|---|---|---|
| FDBF00023 | toluene |
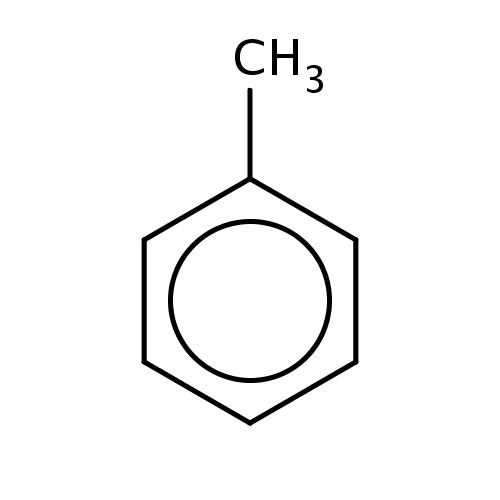
|
c1(ccccc1)C | 0.1268 |
| FDBF00066 | N-methylmethanamine |
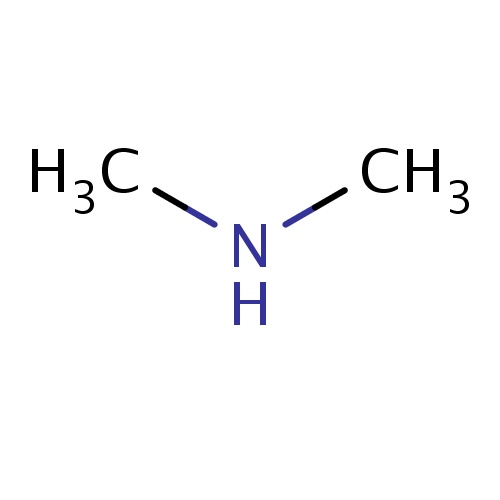
|
N(C)C | 0.0914 |
| FDBF00141 | ethylbenzene |
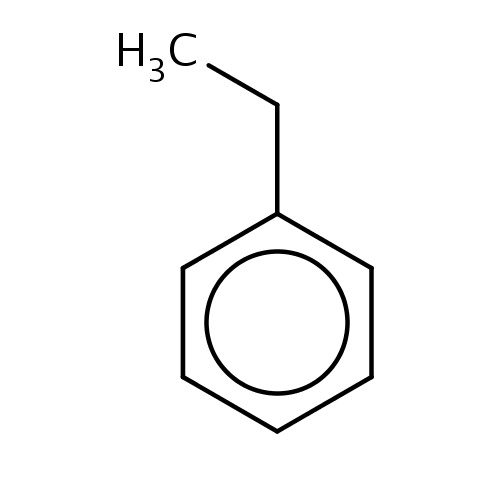
|
c1(ccccc1)CC | 0.0371 |
| FDBF00736 | propanal |
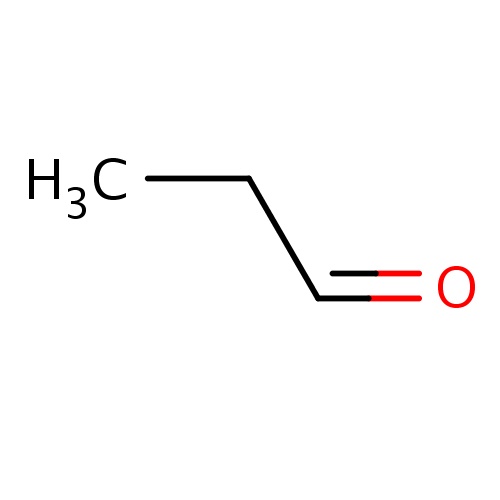
|
O=CCC | 0.0065 |
| FDBF00741 | (2R)-2-phenylpropanal |
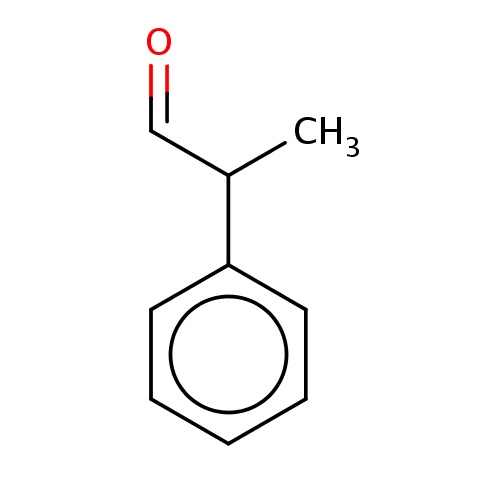
|
O=CC(C)c1ccccc1 | 0.0003 |
| FDBF00742 | pentanal |
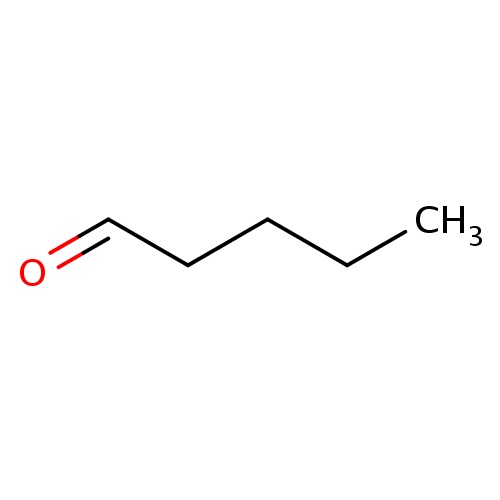
|
O=CCCCC | 0.0003 |
| FDBF00744 | 1-phenylbutan-2-one |
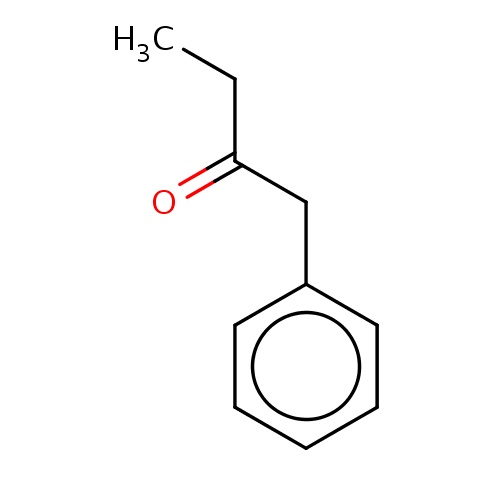
|
O=C(Cc1ccccc1)CC | 0.0003 |
| FDBF00745 | (2R)-N,N-dimethylbutan-2-amine |
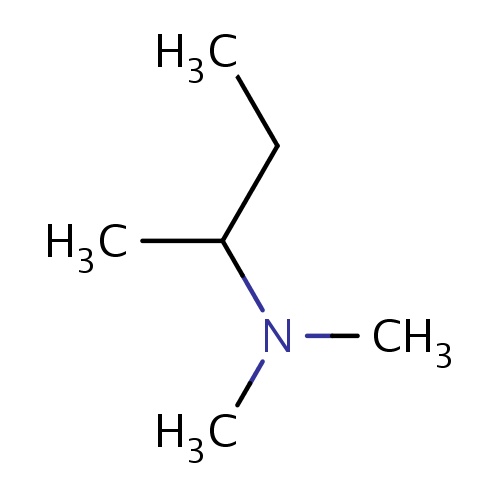
|
N(C)(C)C(CC)C | 0.0007 |

