IUPAC name
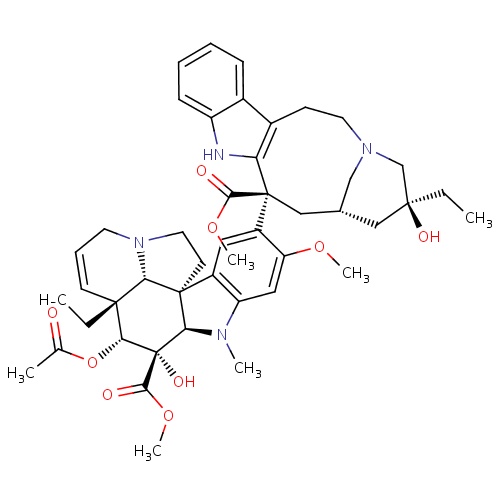
methyl (1R,9R,10S,11R,12R,19R)-11-(acetyloxy)-12-ethyl-4-[(13S,15S,17S)-17-ethyl-17-hydroxy-13-(methoxycarbonyl)-1,11-diazatetracyclo[13.3.1.0
SMILES
[H][C@@]12N(C)C3=C(C=C(C(OC)=C3)[C@]3(C[C@@H]4CN(C[C@](O)(CC)C4)CCC4=C3NC3=CC=CC=C43)C(=O)OC)[C@@]11CCN3CC=C[C@@](CC)([C@@H](OC(C)=O)[C@]2(O)C(=O)OC)[C@@]13[H]
Compound class
Antineoplastic Agents; Immunosuppressive Agents; Antineoplastic Agents, Phytogenic; Tubulin Modulators; Antineoplastic and Immunomodulating Agents; Vinca Alkaloids and Analogues; CYP2D6 Inducers; CYP2D6 Inducers (strong); CYP3A4 Inhibitors; BSEP/ABCB11 Inhibitors; Combined Inhibitors of CYP3A4 and P-glycoprotein;
Therapeutic area
For treatment of breast cancer, testicular cancer, lymphomas, neuroblastoma, Hodgkin's and non-Hodgkin's lymphomas, mycosis fungoides, histiocytosis, and Kaposi's sarcoma.
Common name
Vinblastine
IUPAC name
methyl (1R,9R,10S,11R,12R,19R)-11-(acetyloxy)-12-ethyl-4-[(13S,15S,17S)-17-ethyl-17-hydroxy-13-(methoxycarbonyl)-1,11-diazatetracyclo[13.3.1.0
SMILES
[H][C@@]12N(C)C3=C(C=C(C(OC)=C3)[C@]3(C[C@@H]4CN(C[C@](O)(CC)C4)CCC4=C3NC3=CC=CC=C43)C(=O)OC)[C@@]11CCN3CC=C[C@@](CC)([C@@H](OC(C)=O)[C@]2(O)C(=O)OC)[C@@]13[H]
INCHI
InChI=1S/C46H58N4O9/c1-8-42(54)23-28-24-45(40(52)57-6,36-30(15-19-49(25-28)26-42)29-13-10-11-14-33(29)47-36)32-21-31-34(22-35(32)56-5)48(4)38-44(31)17-20-50-18-12-16-43(9-2,37(44)50)39(59-27(3)51)46(38,55)41(53)58-7/h10-14,16,21-22,28,37-39,47,54-55H,8-9,15,17-20,23-26H2,1-7H3/t28-,37+,38-,39-,42+,43-,44-,45+,46+/m1/s1
FORMULA
C46H58N4O9

Common name
Vinblastine
IUPAC name
methyl (1R,9R,10S,11R,12R,19R)-11-(acetyloxy)-12-ethyl-4-[(13S,15S,17S)-17-ethyl-17-hydroxy-13-(methoxycarbonyl)-1,11-diazatetracyclo[13.3.1.0
Molecular weight
810.974
clogP
4.724
clogS
-7.554
HBond Acceptor
12
HBond Donor
3
Total Polar
Surface Area
154.1
Number of Rings
9
Rotatable Bond
10