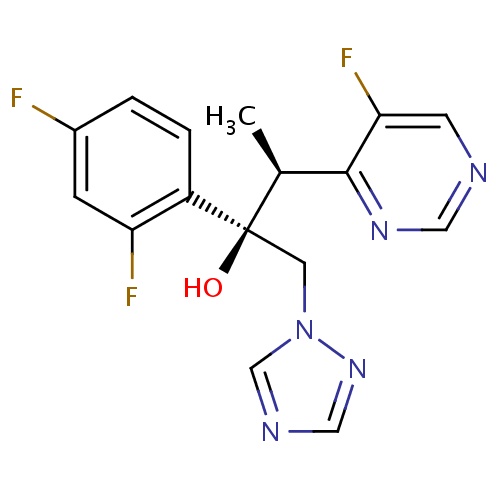
IUPAC name
(2R,3S)-2-(2,4-difluorophenyl)-3-(5-fluoropyrimidin-4-yl)-1-(1H-1,2,4-triazol-1-yl)butan-2-ol
SMILES
C[C@@H](C1=NC=NC=C1F)[C@](O)(CN1C=NC=N1)C1=C(F)C=C(F)C=C1
Compound class
Antifungal Agents; 14-alpha Demethylase Inhibitors; Antiinfectives for Systemic Use; Triazole Derivatives; Antimycotics for Systemic Use; Cytochrome P-450 CYP2C9 Inhibitors; Cytochrome P-450 CYP3A Inhibitors; Cytochrome P-450 CYP2C9 Inducers; Cytochrome P-450 CYP2C19 Inducers; CYP3A4 Inhibitors;
Therapeutic area
For the treatment of esophageal candidiasis, invasive pulmonary aspergillosis, and serious fungal infections caused by .
Common name
Voriconazole
IUPAC name
(2R,3S)-2-(2,4-difluorophenyl)-3-(5-fluoropyrimidin-4-yl)-1-(1H-1,2,4-triazol-1-yl)butan-2-ol
SMILES
C[C@@H](C1=NC=NC=C1F)[C@](O)(CN1C=NC=N1)C1=C(F)C=C(F)C=C1
INCHI
InChI=1S/C16H14F3N5O/c1-10(15-14(19)5-20-7-22-15)16(25,6-24-9-21-8-23-24)12-3-2-11(17)4-13(12)18/h2-5,7-10,25H,6H2,1H3/t10-,16+/m0/s1
FORMULA
C16H14F3N5O

Common name
Voriconazole
IUPAC name
(2R,3S)-2-(2,4-difluorophenyl)-3-(5-fluoropyrimidin-4-yl)-1-(1H-1,2,4-triazol-1-yl)butan-2-ol
Molecular weight
349.310
clogP
2.833
clogS
-4.380
HBond Acceptor
5
HBond Donor
1
Total Polar
Surface Area
76.72
Number of Rings
3
Rotatable Bond
5
| Drug ID | Common name | Structure CAS | SMILE | Frequency |
|---|---|---|---|---|
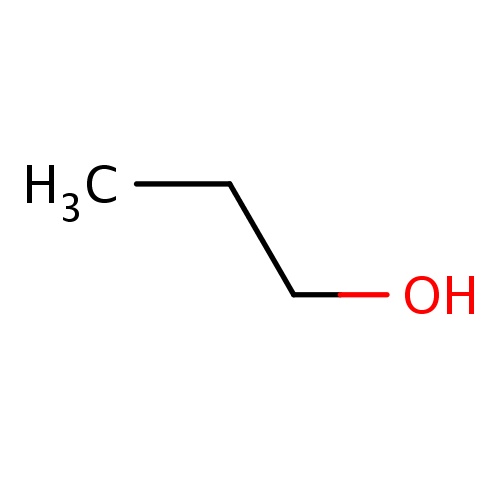
| FDBF00018 | propan-1-ol |
|
C(O)CC | 0.0330 |
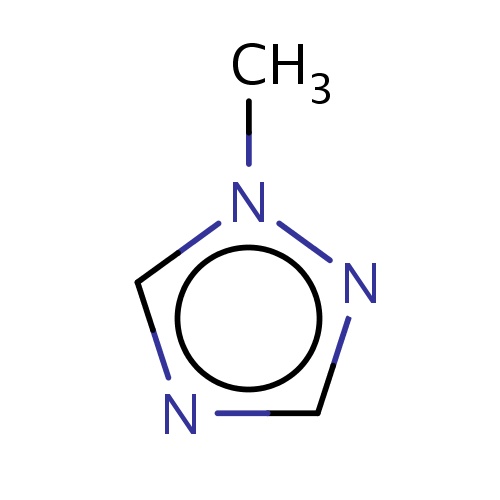
| FDBF00261 | 1-methyl-1,2,4-triazole |
|
n1(ncnc1)C | 0.0038 |
| FDBF00269 | 1,3-difluorobenzene |

|
Fc1cccc(c1)F | 0.0072 |
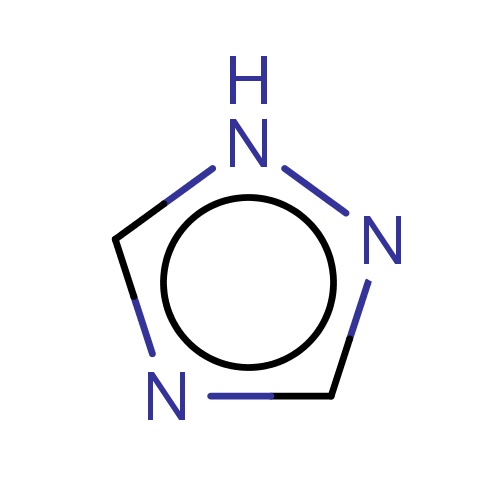
| FDBF00270 | 1H-1,2,4-triazole |
|
[nH]1ncnc1 | 0.0045 |
| FDBF00406 | (2R)-butan-2-ol |

|
CCC(C)O | 0.0055 |
| FDBF01302 | (2S)-2-(5-fluoropyrimidin-4-yl)propan-1-ol |

|
C(O)C(C)c1c(cncn1)F | 0.0003 |
| FDBF01303 | (2S)-1-(1,2,4-triazol-1-yl)butan-2-ol |

|
C(C(O)CC)n1ncnc1 | 0.0010 |
| FDBF01304 | (2R,3S)-3-(5-fluoropyrimidin-4-yl)butan-2-ol |

|
CC(O)C(C)c1c(cncn1)F | 0.0003 |
| FDBF01305 | 5-fluoropyrimidine |

|
Fc1cncnc1 | 0.0007 |

