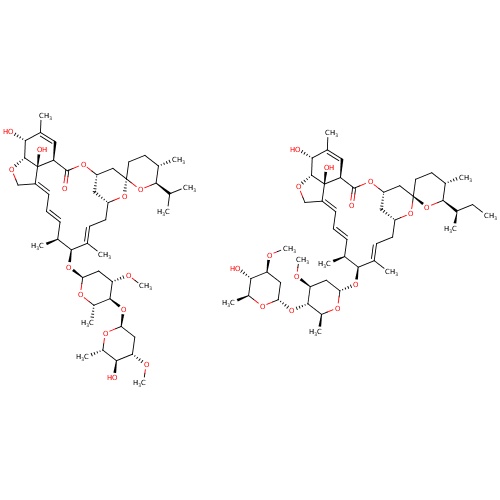
IUPAC name
(1'R,2R,4'S,5S,6R,8'R,10'E,12'S,13'S,14'E,16'E,20'R,21'R,24'S)-21',24'-dihydroxy-12'-{[(2R,4S,5S,6S)-5-{[(2S,4S,5S,6S)-5-hydroxy-4-methoxy-6-methyloxan-2-yl]oxy}-4-methoxy-6-methyloxan-2-yl]oxy}-5,11',13',22'-tetramethyl-6-(propan-2-yl)-3',7',19'-trioxaspiro[oxane-2,6'-tetracyclo[15.6.1.1
SMILES
CO[C@H]1C[C@@H](O[C@@H](C)[C@@H]1O)O[C@H]1[C@H](C)O[C@H](C[C@@H]1OC)O[C@H]1[C@@H](C)\C=C\C=C2/CO[C@@H]3[C@H](O)C(C)=C[C@@H](C(=O)O[C@H]4C[C@@H](C\C=C1/C)O[C@@]1(CC[C@H](C)[C@@H](C(C)C)O1)C4)[C@]23O.CC[C@@H](C)[C@H]1O[C@@]2(CC[C@@H]1C)O[C@@H]1C\C=C(C)\[C@@H](O[C@@H]3O[C@@H](C)[C@H](O[C@@H]4O[C@@H](C)[C@H](O)[C@@H](OC)C4)[C@@H](OC)C3)[C@@H](C)\C=C\C=C3/CO[C@@H]4[C@H](O)C(C)=C[C@@H](C(=O)O[C@@H](C1)C2)[C@]34O
Compound class
Insecticides; Antiparasitic Agents; Antinematodal Agents; Anthelmintics; Sensory Organs; Dermatologicals; Antiparasitic Products, Insecticides and Repellents; Otologicals; Antiparasitics; Avermectins; Macrocyclic Lactones; Endectocides; CYP3A4 Inhibitors;
Therapeutic area
For the treatment of intestinal (i.e., nondisseminated) strongyloidiasis due to the nematode parasite .
Common name
Ivermectin
IUPAC name
(1'R,2R,4'S,5S,6R,8'R,10'E,12'S,13'S,14'E,16'E,20'R,21'R,24'S)-21',24'-dihydroxy-12'-{[(2R,4S,5S,6S)-5-{[(2S,4S,5S,6S)-5-hydroxy-4-methoxy-6-methyloxan-2-yl]oxy}-4-methoxy-6-methyloxan-2-yl]oxy}-5,11',13',22'-tetramethyl-6-(propan-2-yl)-3',7',19'-trioxaspiro[oxane-2,6'-tetracyclo[15.6.1.1
SMILES
CO[C@H]1C[C@@H](O[C@@H](C)[C@@H]1O)O[C@H]1[C@H](C)O[C@H](C[C@@H]1OC)O[C@H]1[C@@H](C)\C=C\C=C2/CO[C@@H]3[C@H](O)C(C)=C[C@@H](C(=O)O[C@H]4C[C@@H](C\C=C1/C)O[C@@]1(CC[C@H](C)[C@@H](C(C)C)O1)C4)[C@]23O.CC[C@@H](C)[C@H]1O[C@@]2(CC[C@@H]1C)O[C@@H]1C\C=C(C)\[C@@H](O[C@@H]3O[C@@H](C)[C@H](O[C@@H]4O[C@@H](C)[C@H](O)[C@@H](OC)C4)[C@@H](OC)C3)[C@@H](C)\C=C\C=C3/CO[C@@H]4[C@H](O)C(C)=C[C@@H](C(=O)O[C@@H](C1)C2)[C@]34O
INCHI
InChI=1S/C48H74O14.C47H72O14/c1-11-25(2)43-28(5)17-18-47(62-43)23-34-20-33(61-47)16-15-27(4)42(26(3)13-12-14-32-24-55-45-40(49)29(6)19-35(46(51)58-34)48(32,45)52)59-39-22-37(54-10)44(31(8)57-39)60-38-21-36(53-9)41(50)30(7)56-38;1-24(2)41-27(5)16-17-46(61-41)22-33-19-32(60-46)15-14-26(4)42(25(3)12-11-13-31-23-54-44-39(48)28(6)18-34(45(50)57-33)47(31,44)51)58-38-21-36(53-10)43(30(8)56-38)59-37-20-35(52-9)40(49)29(7)55-37/h12-15,19,25-26,28,30-31,33-45,49-50,52H,11,16-18,20-24H2,1-10H3;11-14,18,24-25,27,29-30,32-44,48-49,51H,15-17,19-23H2,1-10H3/b13-12+,27-15+,32-14+;12-11+,26-14+,31-13+/t25-,26+,28+,30+,31+,33-,34+,35+,36+,37+,38+,39+,40-,41+,42+,43-,44+,45-,47-,48-;25-,27-,29-,30-,32+,33-,34-,35-,36-,37-,38-,39+,40-,41+,42-,43-,44+,46+,47+/m10/s1
FORMULA
C95H146O28

Common name
Ivermectin
IUPAC name
(1'R,2R,4'S,5S,6R,8'R,10'E,12'S,13'S,14'E,16'E,20'R,21'R,24'S)-21',24'-dihydroxy-12'-{[(2R,4S,5S,6S)-5-{[(2S,4S,5S,6S)-5-hydroxy-4-methoxy-6-methyloxan-2-yl]oxy}-4-methoxy-6-methyloxan-2-yl]oxy}-5,11',13',22'-tetramethyl-6-(propan-2-yl)-3',7',19'-trioxaspiro[oxane-2,6'-tetracyclo[15.6.1.1
Molecular weight
875.093
clogP
2.718
clogS
-3.889
HBond Acceptor
14
HBond Donor
3
Total Polar Surface Area
170.06
Number of Rings
7
Rotatable Bond
8
| Drug ID | Common name | Structure CAS | SMILE | Frequency |
|---|---|---|---|---|
| FDBF01160 | (2S,5R,6S)-6-methyltetrahydropyran-2,5-diol |

|
C1(OC(C(CC1)O)C)O | 0.0010 |
| FDBF01161 | (2S,3R)-2-methyltetrahydropyran-3-ol |

|
C1OC(C(CC1)O)C | 0.0027 |
| FDBF01344 | (2R,3R,4R)-4-methoxy-2-methyl-tetrahydropyran-3-ol |

|
COC1CCOC(C1O)C | 0.0003 |
| FDBF01346 | (2S,4S)-4-methoxy-2-methyl-tetrahydropyran |

|
C1CC(CC(O1)C)OC | 0.0027 |
| FDBF01348 | (2R,6R)-6-methyltetrahydropyran-2-ol |

|
C1C(OC(CC1)O)C | 0.0014 |
| FDBF01349 | (2R,4S)-4-methoxy-2-methyl-tetrahydropyran |

|
C1C(OCCC1OC)C | 0.0003 |
| FDBF01351 | (2R,4R,6R)-4-methoxy-6-methyl-tetrahydropyran-2-ol |

|
C1C(OC(CC1OC)O)C | 0.0003 |
| FDBF01352 | (2S,4R,6S)-4-methoxy-6-methyl-tetrahydropyran-2-ol |

|
OC1CC(CC(O1)C)OC | 0.0007 |
| FDBF01353 | (2S)-2-methyltetrahydropyran |

|
C1CCCC(O1)C | 0.0034 |
| FDBF01354 | (2R)-2-methyltetrahydropyran |

|
C1C(OCCC1)C | 0.0017 |

