IUPAC name
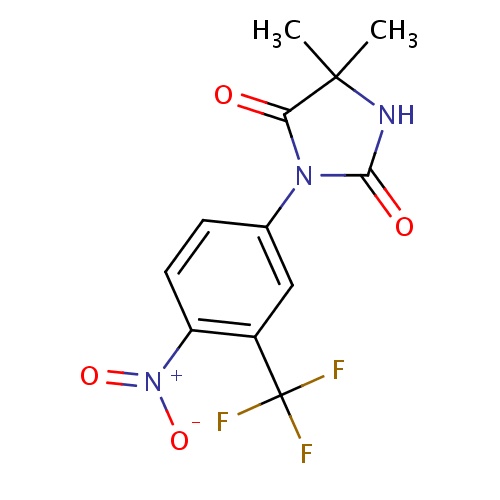
5,5-dimethyl-3-[4-nitro-3-(trifluoromethyl)phenyl]imidazolidine-2,4-dione
SMILES
CC1(C)NC(=O)N(C1=O)C1=CC(=C(C=C1)[N+]([O-])=O)C(F)(F)F
Compound class
Antineoplastic Agents; Androgen Antagonists; Antineoplastic and Immunomodulating Agents; Endocrine Therapy; Hormone Antagonists and Related Agents; Anti-Androgens; Cytochrome P-450 CYP2C9 Inhibitors; Cytochrome P-450 CYP2C8 Inhibitors; Cytochrome P-450 CYP2C9 Inducers; Cytochrome P-450 CYP2C19 Inducers; Cytochrome P-450 CYP2C8 Inducers;
Therapeutic area
For use in combination with surgical castration for the treatment of metastatic prostate cancer involving distant lymph nodes, bone, or visceral organs (Stage D2).
Common name
Nilutamide
IUPAC name
5,5-dimethyl-3-[4-nitro-3-(trifluoromethyl)phenyl]imidazolidine-2,4-dione
SMILES
CC1(C)NC(=O)N(C1=O)C1=CC(=C(C=C1)[N+]([O-])=O)C(F)(F)F
INCHI
InChI=1S/C12H10F3N3O4/c1-11(2)9(19)17(10(20)16-11)6-3-4-8(18(21)22)7(5-6)12(13,14)15/h3-5H,1-2H3,(H,16,20)
FORMULA
C12H10F3N3O4

Common name
Nilutamide
IUPAC name
5,5-dimethyl-3-[4-nitro-3-(trifluoromethyl)phenyl]imidazolidine-2,4-dione
Molecular weight
317.221
clogP
1.255
clogS
-3.577
HBond Acceptor
4
HBond Donor
1
Total Polar Surface Area
101.22
Number of Rings
2
Rotatable Bond
3