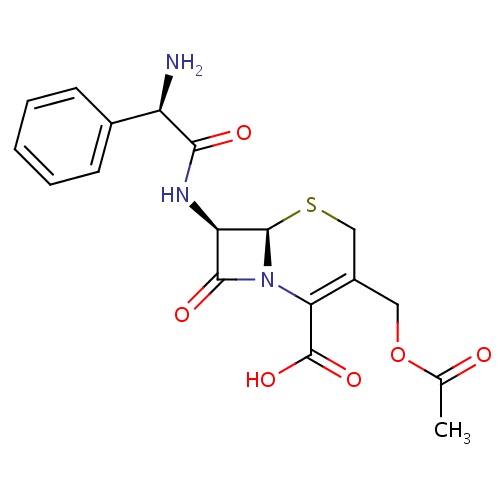
IUPAC name
(6R,7R)-3-[(acetyloxy)methyl]-7-[(2R)-2-amino-2-phenylacetamido]-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic acid
SMILES
[H][C@]12SCC(COC(C)=O)=C(N1C(=O)[C@H]2NC(=O)[C@H](N)C1=CC=CC=C1)C(O)=O
Compound class
Anti-Bacterial Agents; Cephalosporins;
Therapeutic area
For treatment of severe infections caused by susceptible bacteria.
Common name
Cephaloglycin
IUPAC name
(6R,7R)-3-[(acetyloxy)methyl]-7-[(2R)-2-amino-2-phenylacetamido]-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic acid
SMILES
[H][C@]12SCC(COC(C)=O)=C(N1C(=O)[C@H]2NC(=O)[C@H](N)C1=CC=CC=C1)C(O)=O
INCHI
InChI=1S/C18H19N3O6S/c1-9(22)27-7-11-8-28-17-13(16(24)21(17)14(11)18(25)26)20-15(23)12(19)10-5-3-2-4-6-10/h2-6,12-13,17H,7-8,19H2,1H3,(H,20,23)(H,25,26)/t12-,13-,17-/m1/s1
FORMULA
C18H19N3O6S

Common name
Cephaloglycin
IUPAC name
(6R,7R)-3-[(acetyloxy)methyl]-7-[(2R)-2-amino-2-phenylacetamido]-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic acid
Molecular weight
405.425
clogP
0.032
clogS
-1.608
HBond Acceptor
6
HBond Donor
4
Total Polar Surface Area
164.33
Number of Rings
3
Rotatable Bond
7