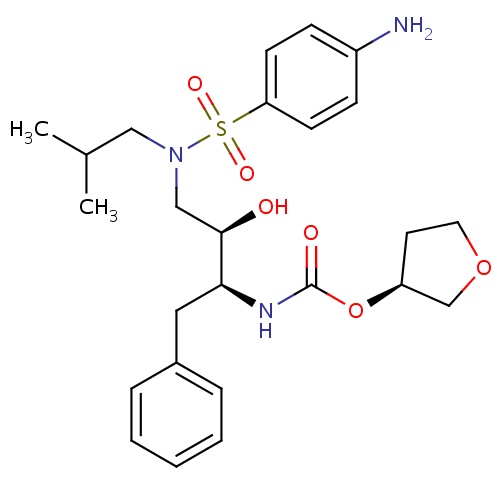
IUPAC name
(3S)-oxolan-3-yl N-[(2S,3R)-3-hydroxy-4-[N-(2-methylpropyl)4-aminobenzenesulfonamido]-1-phenylbutan-2-yl]carbamate
SMILES
CC(C)CN(C[C@@H](O)[C@H](CC1=CC=CC=C1)NC(=O)O[C@H]1CCOC1)S(=O)(=O)C1=CC=C(N)C=C1
Compound class
Anti-HIV Agents; Antibiotics, Antitubercular; Protease Inhibitors; HIV Protease Inhibitors; Antiinfectives for Systemic Use; Direct Acting Antivirals; Antivirals for Systemic Use; Cytochrome P-450 CYP2C9 Inhibitors; Cytochrome P-450 CYP2C8 Inhibitors; Cytochrome P-450 CYP2C9 Inducers; Cytochrome P-450 CYP2C19 Inducers; Cytochrome P-450 CYP2C8 Inducers; Cytochrome P-450 CYP2B6 Inducers; Cytochrome P-450 CYP2B6 Inhibitors; CYP2B6 Inhibitors (strong); CYP2D6 Inducers; CYP2D6 Inducers (strong); CYP3A4 Inhibitors; Combined Inhibitors of CYP3A4 and P-glycoprotein;
Therapeutic area
For the treatment of HIV-1 infection in combination with other antiretroviral agents.
Common name
Amprenavir
IUPAC name
(3S)-oxolan-3-yl N-[(2S,3R)-3-hydroxy-4-[N-(2-methylpropyl)4-aminobenzenesulfonamido]-1-phenylbutan-2-yl]carbamate
SMILES
CC(C)CN(C[C@@H](O)[C@H](CC1=CC=CC=C1)NC(=O)O[C@H]1CCOC1)S(=O)(=O)C1=CC=C(N)C=C1
INCHI
InChI=1S/C25H35N3O6S/c1-18(2)15-28(35(31,32)22-10-8-20(26)9-11-22)16-24(29)23(14-19-6-4-3-5-7-19)27-25(30)34-21-12-13-33-17-21/h3-11,18,21,23-24,29H,12-17,26H2,1-2H3,(H,27,30)/t21-,23-,24+/m0/s1
FORMULA
C25H35N3O6S

Common name
Amprenavir
IUPAC name
(3S)-oxolan-3-yl N-[(2S,3R)-3-hydroxy-4-[N-(2-methylpropyl)4-aminobenzenesulfonamido]-1-phenylbutan-2-yl]carbamate
Molecular weight
505.627
clogP
1.782
clogS
-4.189
HBond Acceptor
6
HBond Donor
4
Total Polar Surface Area
131.19
Number of Rings
3
Rotatable Bond
12
| Drug ID | Common name | Structure CAS | SMILE | Frequency |
|---|---|---|---|---|
| FDBF00005 | benzene |
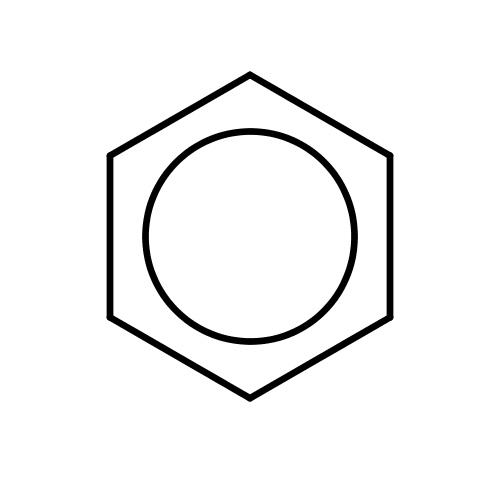
|
c1ccccc1 | 0.2824 |
| FDBF00018 | propan-1-ol |
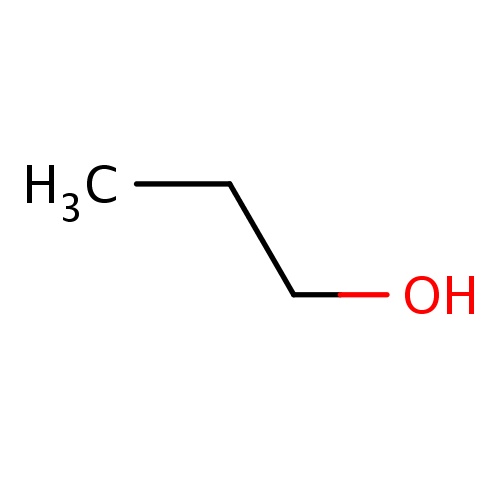
|
C(O)CC | 0.0330 |
| FDBF00023 | toluene |
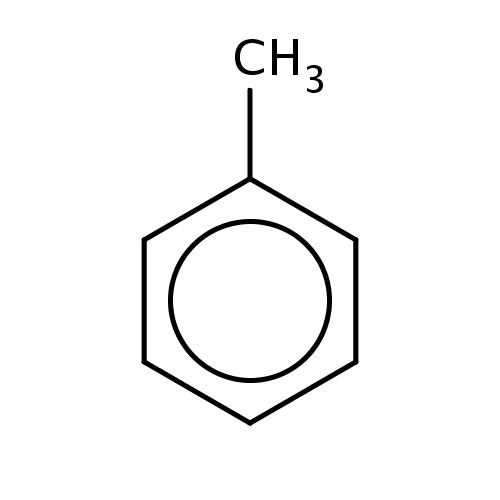
|
c1(ccccc1)C | 0.1268 |
| FDBF00042 | propan-2-ol |
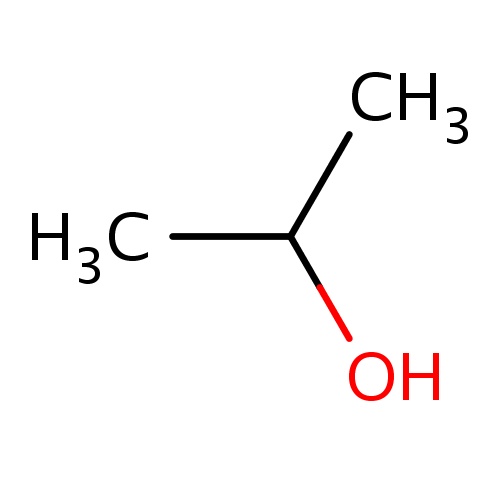
|
CC(O)C | 0.0278 |
| FDBF00141 | ethylbenzene |
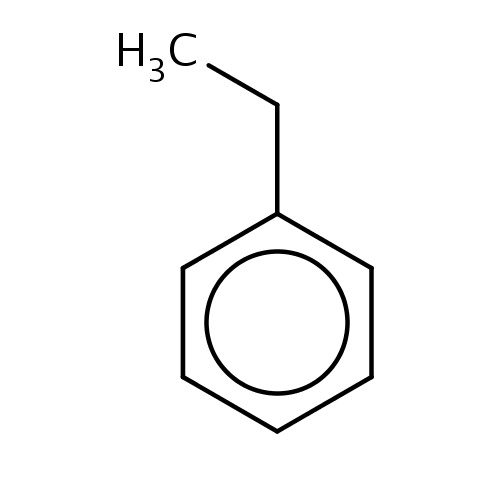
|
c1(ccccc1)CC | 0.0371 |
| FDBF00287 | 2-(methylamino)ethanol |
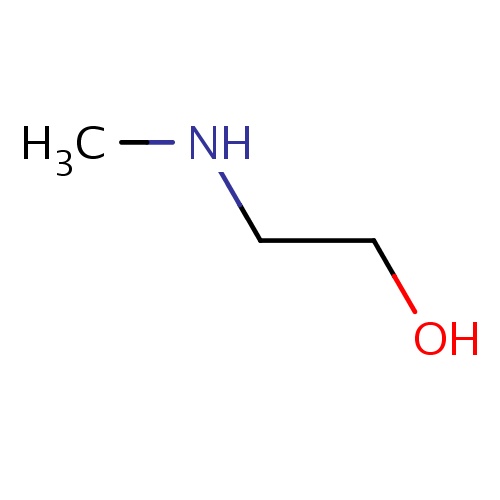
|
C(CO)NC | 0.0089 |
| FDBF01127 | methylcarbamic acid |

|
O=C(O)NC | 0.0199 |
| FDBF01148 | (2R)-4-phenylbutan-2-ol |
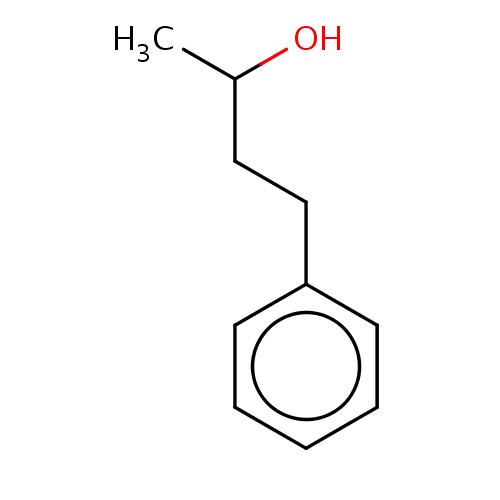
|
c1(ccccc1)CCC(C)O | 0.0021 |
| FDBF01627 | N-(dihydroxy-λ3-sulfanyl)-N-methyl-methanamine |
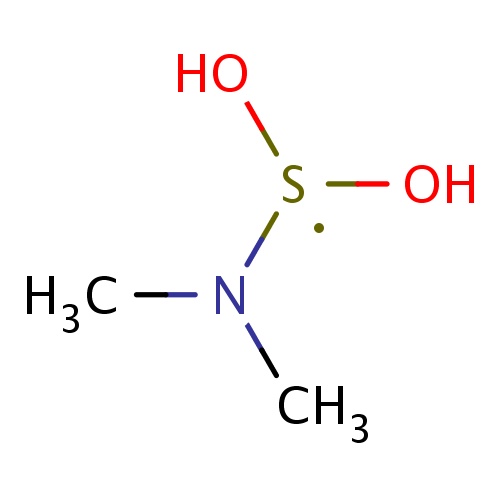
|
[S](O)(O)N(C)C | 0.0041 |
| FDBF01628 | N-(dihydroxy-λ3-sulfanyl)-2-methyl-propan-1-amine |
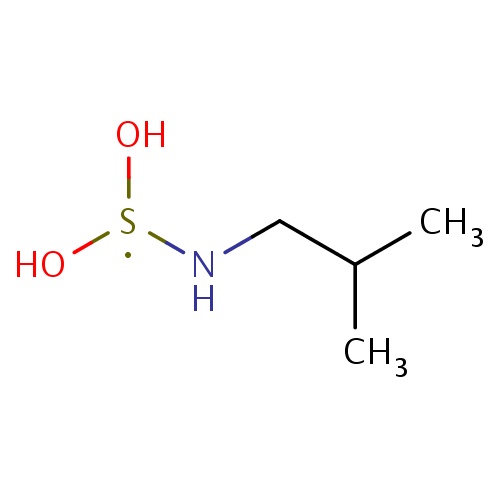
|
[S](O)(O)NCC(C)C | 0.0010 |

