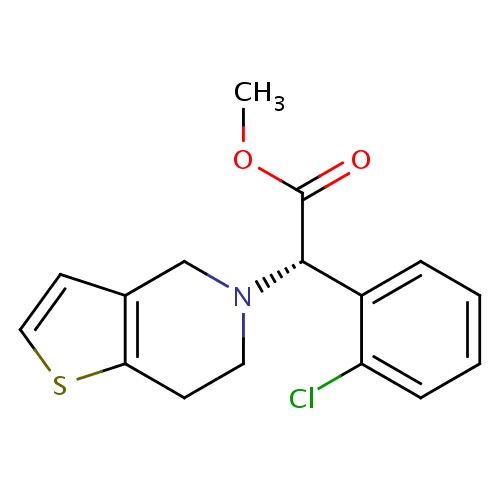
IUPAC name
methyl (2S)-2-(2-chlorophenyl)-2-{4H,5H,6H,7H-thieno[3,2-c]pyridin-5-yl}acetate
SMILES
[H][C@@](N1CCC2=C(C1)C=CS2)(C(=O)OC)C1=CC=CC=C1Cl
Compound class
Platelet Aggregation Inhibitors; Antithrombotic Agents; Blood and Blood Forming Organs; Platelet Aggregation Inhibitors Excl. Heparin; Cytochrome P-450 CYP1A2 Inhibitors; Cytochrome P-450 CYP2C9 Inhibitors; Purinergic P2Y Receptor Antagonists; Cytochrome P-450 CYP1A2 Inducers; Cytochrome P-450 CYP2C8 Inhibitors; Cytochrome P-450 CYP2C9 Inducers; Cytochrome P-450 CYP2C19 Inducers; Cytochrome P-450 CYP2C8 Inducers; Cytochrome P-450 CYP2B6 Inducers; Cytochrome P-450 CYP2B6 Inhibitors; CYP2B6 Inhibitors (strong); CYP3A4 Inhibitors;
Therapeutic area
For the reduction of atherosclerotic events (myocardial infarction, stroke, and vascular death) in patients with atherosclerosis documented by recent stroke, recent myocardial infarction, or established peripheral arterial disease.
Common name
Clopidogrel
IUPAC name
methyl (2S)-2-(2-chlorophenyl)-2-{4H,5H,6H,7H-thieno[3,2-c]pyridin-5-yl}acetate
SMILES
[H][C@@](N1CCC2=C(C1)C=CS2)(C(=O)OC)C1=CC=CC=C1Cl
INCHI
InChI=1S/C16H16ClNO2S/c1-20-16(19)15(12-4-2-3-5-13(12)17)18-8-6-14-11(10-18)7-9-21-14/h2-5,7,9,15H,6,8,10H2,1H3/t15-/m0/s1
FORMULA
C16H16ClNO2S

Common name
Clopidogrel
IUPAC name
methyl (2S)-2-(2-chlorophenyl)-2-{4H,5H,6H,7H-thieno[3,2-c]pyridin-5-yl}acetate
Molecular weight
321.822
clogP
4.622
clogS
-4.161
HBond Acceptor
3
HBond Donor
0
Total Polar Surface Area
57.78
Number of Rings
3
Rotatable Bond
4
| Drug ID | Common name | Structure CAS | SMILE | Frequency |
|---|---|---|---|---|
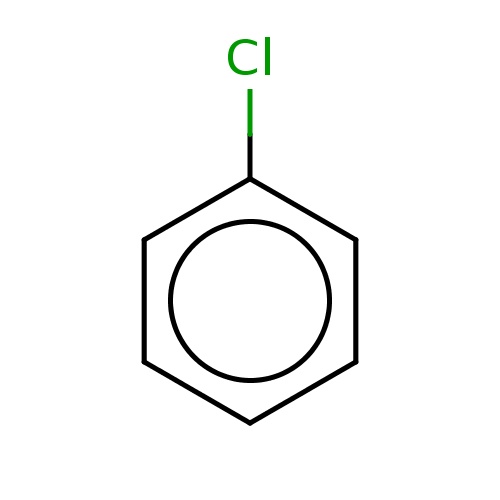
| FDBF00016 | chlorobenzene |
|
c1ccc(cc1)Cl | 0.0718 |
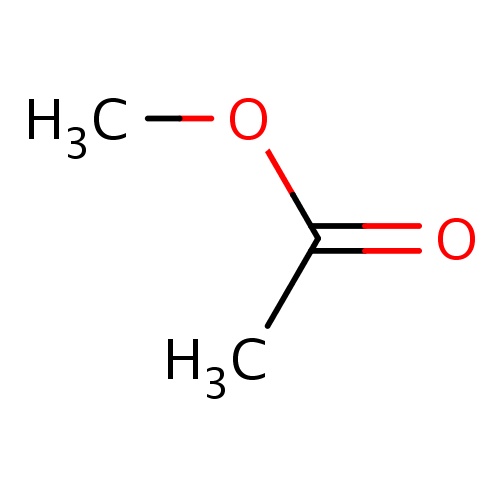
| FDBF00101 | methyl acetate |
|
O(C(=O)C)C | 0.0151 |
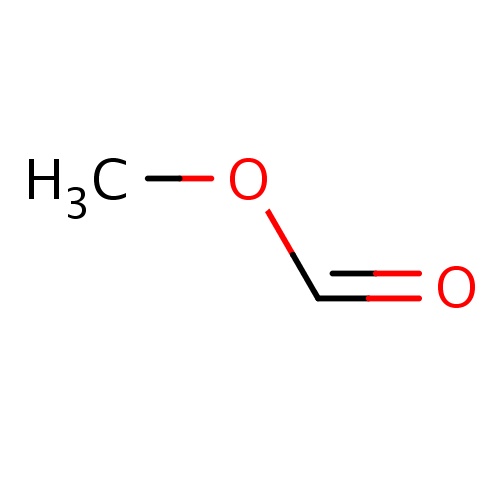
| FDBF00105 | methyl formate |
|
O(C=O)C | 0.0323 |
| FDBF00302 | 1-chloro-2-methyl-benzene |
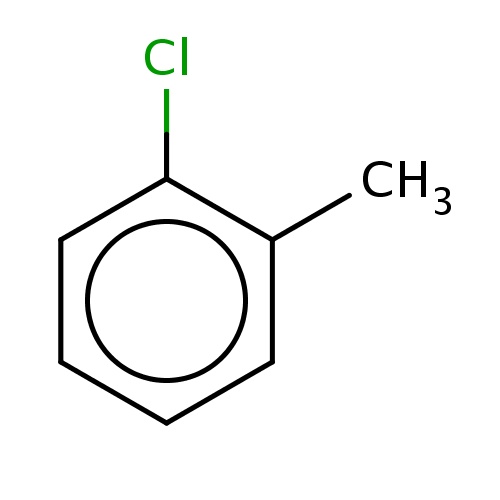
|
Clc1c(cccc1)C | 0.0062 |
| FDBF01742 | methyl 2-(2-chlorophenyl)acetate |

|
O(C(=O)Cc1c(cccc1)Cl)C | 0.0003 |

