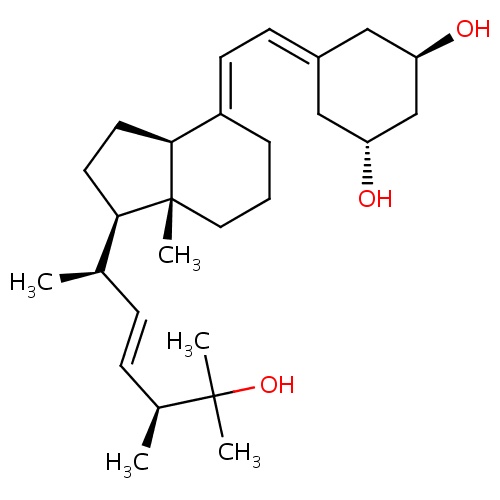
IUPAC name
(1R,3R)-5-{2-[(1R,3aS,4E,7aR)-1-[(2R,3E,5S)-6-hydroxy-5,6-dimethylhept-3-en-2-yl]-7a-methyl-octahydro-1H-inden-4-ylidene]ethylidene}cyclohexane-1,3-diol
SMILES
[H]C1CC[C@]2(C)[C@]([H])(CC[C@@]2([H])\C1=C\C=C1C[C@@H](O)C[C@H](O)C1)[C@H](C)\C=C\[C@H](C)C(O)(C)C
Compound class
Systemic Hormonal Preparations, Excl. Sex Hormones and Insulins; Anti-Parathyroid Agents; Calcium Homeostasis; CYP3A4 Inhibitors;
Therapeutic area
For treatment of secondary hyperparathyroidism associated with chronic kidney disease (CKD) Stage 3 and 4.
Common name
Paricalcitol
IUPAC name
(1R,3R)-5-{2-[(1R,3aS,4E,7aR)-1-[(2R,3E,5S)-6-hydroxy-5,6-dimethylhept-3-en-2-yl]-7a-methyl-octahydro-1H-inden-4-ylidene]ethylidene}cyclohexane-1,3-diol
SMILES
[H]C1CC[C@]2(C)[C@]([H])(CC[C@@]2([H])\C1=C\C=C1C[C@@H](O)C[C@H](O)C1)[C@H](C)\C=C\[C@H](C)C(O)(C)C
INCHI
InChI=1S/C27H44O3/c1-18(8-9-19(2)26(3,4)30)24-12-13-25-21(7-6-14-27(24,25)5)11-10-20-15-22(28)17-23(29)16-20/h8-11,18-19,22-25,28-30H,6-7,12-17H2,1-5H3/b9-8+,21-11+/t18-,19+,22-,23-,24-,25+,27-/m1/s1
FORMULA
C27H44O3

Common name
Paricalcitol
IUPAC name
(1R,3R)-5-{2-[(1R,3aS,4E,7aR)-1-[(2R,3E,5S)-6-hydroxy-5,6-dimethylhept-3-en-2-yl]-7a-methyl-octahydro-1H-inden-4-ylidene]ethylidene}cyclohexane-1,3-diol
Molecular weight
416.636
clogP
5.469
clogS
-3.525
HBond Acceptor
3
HBond Donor
3
Total Polar Surface Area
60.69
Number of Rings
3
Rotatable Bond
5