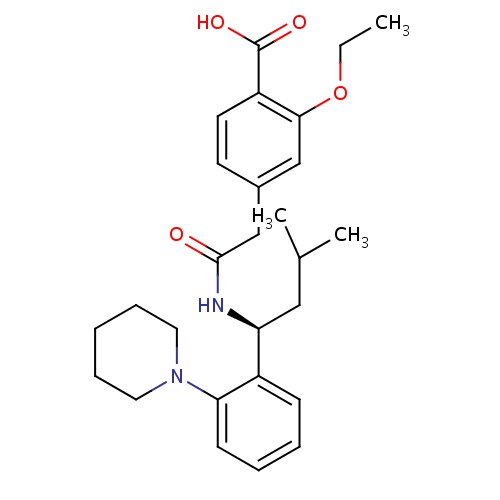
IUPAC name
2-ethoxy-4-({[(1S)-3-methyl-1-[2-(piperidin-1-yl)phenyl]butyl]carbamoyl}methyl)benzoic acid
SMILES
CCOC1=C(C=CC(CC(=O)N[C@@H](CC(C)C)C2=CC=CC=C2N2CCCCC2)=C1)C(O)=O
Compound class
Hypoglycemic Agents; Antidiabetic Agents; Meglitinides; Drugs Used in Diabetes; Alimentary Tract and Metabolism; Blood Glucose Lowering Drugs, Excl. Insulins; Cytochrome P-450 CYP2C8 Inhibitors; Cytochrome P-450 CYP2C8 Inducers; CYP3A4 Inhibitors;
Therapeutic area
As an adjunct to diet and exercise to improve glycemic control in adults with type 2 diabetes mellitus.
Common name
Repaglinide
IUPAC name
2-ethoxy-4-({[(1S)-3-methyl-1-[2-(piperidin-1-yl)phenyl]butyl]carbamoyl}methyl)benzoic acid
SMILES
CCOC1=C(C=CC(CC(=O)N[C@@H](CC(C)C)C2=CC=CC=C2N2CCCCC2)=C1)C(O)=O
INCHI
InChI=1S/C27H36N2O4/c1-4-33-25-17-20(12-13-22(25)27(31)32)18-26(30)28-23(16-19(2)3)21-10-6-7-11-24(21)29-14-8-5-9-15-29/h6-7,10-13,17,19,23H,4-5,8-9,14-16,18H2,1-3H3,(H,28,30)(H,31,32)/t23-/m0/s1
FORMULA
C27H36N2O4

Common name
Repaglinide
IUPAC name
2-ethoxy-4-({[(1S)-3-methyl-1-[2-(piperidin-1-yl)phenyl]butyl]carbamoyl}methyl)benzoic acid
Molecular weight
452.586
clogP
5.123
clogS
-6.039
HBond Acceptor
5
HBond Donor
2
Total Polar Surface Area
78.87
Number of Rings
3
Rotatable Bond
10
| Drug ID | Common name | Structure CAS | SMILE | Frequency |
|---|---|---|---|---|
| FDBF00005 | benzene |
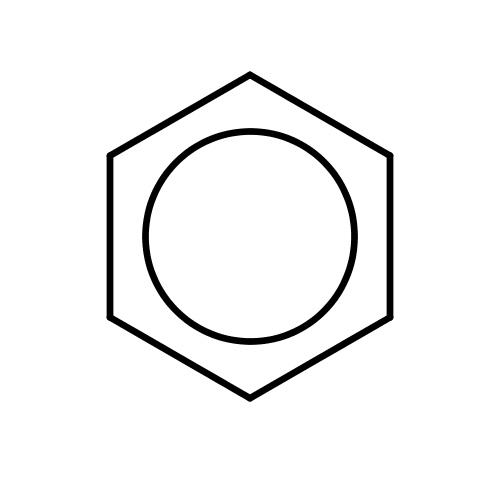
|
c1ccccc1 | 0.2824 |
| FDBF00007 | propane |

|
C(C)C | 0.2412 |
| FDBF00228 | N-ethylacetamide |
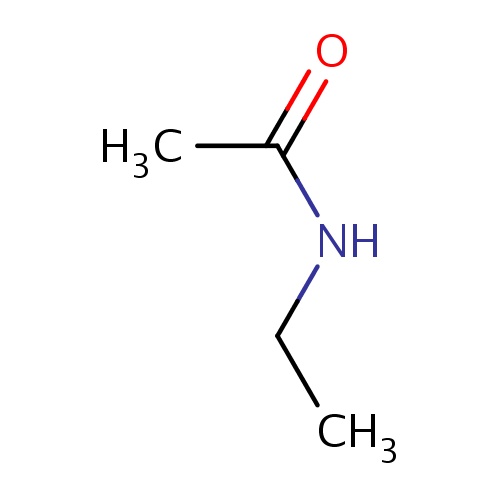
|
C(=O)(NCC)C | 0.0089 |
| FDBF00229 | N-isopentylformamide |

|
C(C)(C)CCNC=O | 0.0010 |
| FDBF00691 | benzoic acid |

|
c1cc(ccc1)C(=O)O | 0.0117 |
| FDBF01808 | 2-hydroxybenzoic acid |
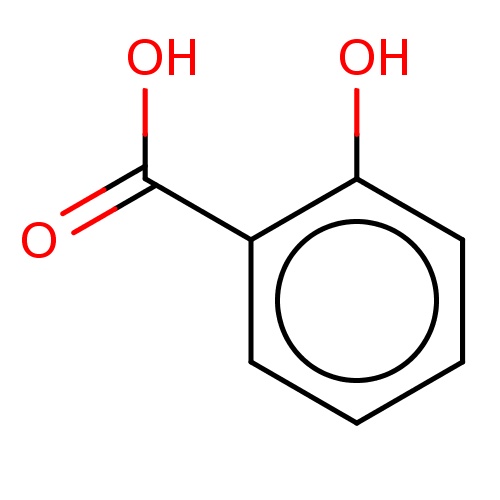
|
c1cc(c(cc1)O)C(=O)O | 0.0041 |
| FDBF02022 | 1-phenylpiperidine |

|
c1(ccccc1)N2CCCCC2 | 0.0003 |
| FDBF02026 | N-[(1S)-1-phenylethyl]formamide |

|
c1c(cccc1)C(C)NC=O | 0.0003 |
| FDBF02027 | 2-ethoxy-4-methyl-benzoic acid |

|
Cc1cc(c(cc1)C(=O)O)OCC | 0.0003 |
| FDBF02031 | N-[(1S)-1-[2-(1-piperidyl)phenyl]ethyl]acetamide |

|
CC(=O)NC(c1c(cccc1)N2CCCCC2)C | 0.0003 |

