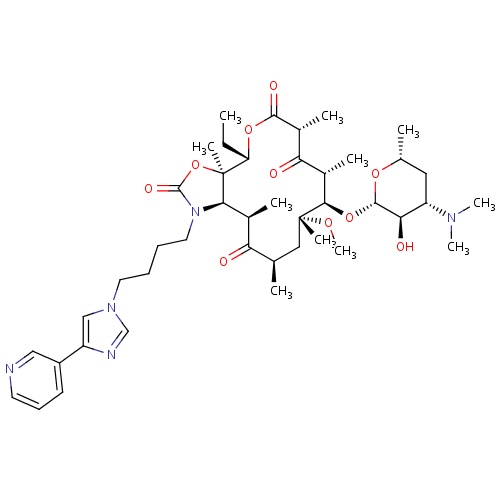
IUPAC name
(3aR,4S,7R,9R,10R,11R,13R,15R,15aR)-10-{[(2S,3R,4S,6R)-4-(dimethylamino)-3-hydroxy-6-methyloxan-2-yl]oxy}-4-ethyl-11-methoxy-3a,7,9,11,13,15-hexamethyl-1-{4-[4-(pyridin-3-yl)-1H-imidazol-1-yl]butyl}-tetradecahydro-1H-oxacyclotetradeca[4,3-d][1,3]oxazole-2,6,8,14-tetrone
SMILES
CC[C@@H]1OC(=O)[C@H](C)C(=O)[C@H](C)[C@@H](O[C@@H]2O[C@H](C)C[C@@H]([C@H]2O)N(C)C)[C@@](C)(C[C@@H](C)C(=O)[C@H](C)[C@H]2N(CCCCN3C=NC(=C3)C3=CC=CN=C3)C(=O)O[C@@]12C)OC
Compound class
Anti-Bacterial Agents; Ketolides; Macrolides; Antibacterials for Systemic Use; Antiinfectives for Systemic Use; Macrolides, Lincosamides and Streptogramins; Cytochrome P-450 CYP1A2 Inhibitors; Cytochrome P-450 CYP1A2 Inducers; CYP2D6 Inducers; CYP2D6 Inducers (strong); CYP3A4 Inhibitors;
Therapeutic area
For the treatment of .
Common name
Telithromycin
IUPAC name
(3aR,4S,7R,9R,10R,11R,13R,15R,15aR)-10-{[(2S,3R,4S,6R)-4-(dimethylamino)-3-hydroxy-6-methyloxan-2-yl]oxy}-4-ethyl-11-methoxy-3a,7,9,11,13,15-hexamethyl-1-{4-[4-(pyridin-3-yl)-1H-imidazol-1-yl]butyl}-tetradecahydro-1H-oxacyclotetradeca[4,3-d][1,3]oxazole-2,6,8,14-tetrone
SMILES
CC[C@@H]1OC(=O)[C@H](C)C(=O)[C@H](C)[C@@H](O[C@@H]2O[C@H](C)C[C@@H]([C@H]2O)N(C)C)[C@@](C)(C[C@@H](C)C(=O)[C@H](C)[C@H]2N(CCCCN3C=NC(=C3)C3=CC=CN=C3)C(=O)O[C@@]12C)OC
INCHI
InChI=1S/C43H65N5O10/c1-12-33-43(8)37(48(41(53)58-43)19-14-13-18-47-23-31(45-24-47)30-16-15-17-44-22-30)27(4)34(49)25(2)21-42(7,54-11)38(28(5)35(50)29(6)39(52)56-33)57-40-36(51)32(46(9)10)20-26(3)55-40/h15-17,22-29,32-33,36-38,40,51H,12-14,18-21H2,1-11H3/t25-,26-,27+,28+,29-,32+,33+,36-,37-,38-,40+,42-,43+/m1/s1
FORMULA
C43H65N5O10

Common name
Telithromycin
IUPAC name
(3aR,4S,7R,9R,10R,11R,13R,15R,15aR)-10-{[(2S,3R,4S,6R)-4-(dimethylamino)-3-hydroxy-6-methyloxan-2-yl]oxy}-4-ethyl-11-methoxy-3a,7,9,11,13,15-hexamethyl-1-{4-[4-(pyridin-3-yl)-1H-imidazol-1-yl]butyl}-tetradecahydro-1H-oxacyclotetradeca[4,3-d][1,3]oxazole-2,6,8,14-tetrone
Molecular weight
812.004
clogP
3.043
clogS
-6.389
HBond Acceptor
13
HBond Donor
1
Total Polar Surface Area
171.85
Number of Rings
5
Rotatable Bond
11
| Drug ID | Common name | Structure CAS | SMILE | Frequency |
|---|---|---|---|---|
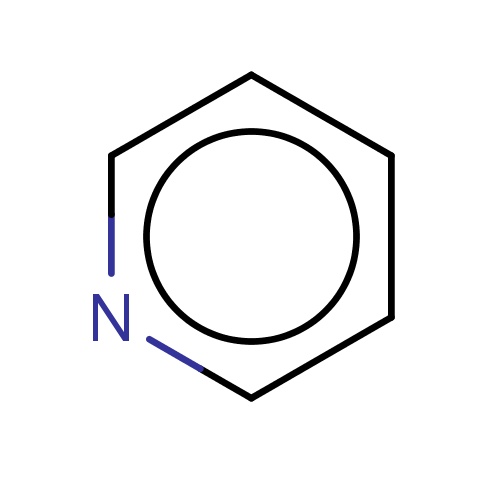
| FDBF00014 | pyridine |
|
c1cccnc1 | 0.0333 |
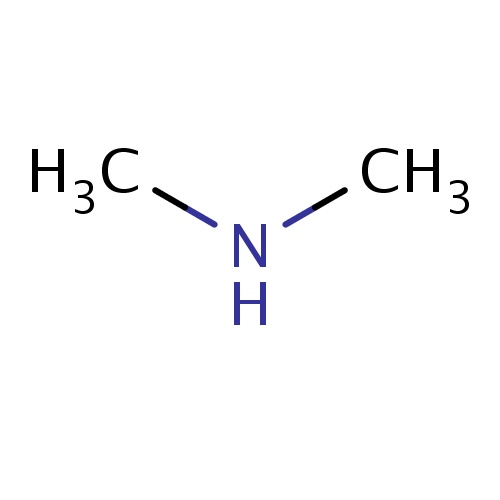
| FDBF00066 | N-methylmethanamine |
|
N(C)C | 0.0914 |
| FDBF00067 | butane |
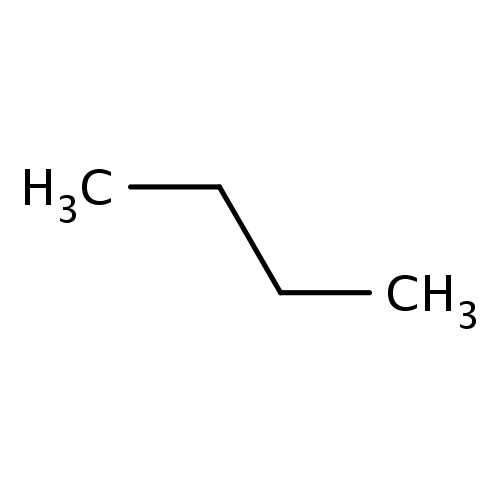
|
CCCC | 0.0680 |
| FDBF02214 | (3S,4R,6S)-4-(dimethylamino)-6-methyl-tetrahydropyran-3-ol |
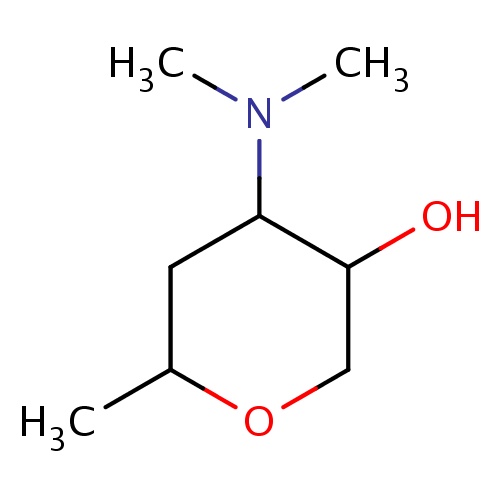
|
N(C)(C)C1C(COC(C1)C)O | 0.0007 |
| FDBF02216 | 3-(1-ethylimidazol-4-yl)pyridine |

|
n1(cc(nc1)c2cnccc2)CC | 0.0003 |
| FDBF02217 | 3-(1-propylimidazol-4-yl)pyridine |

|
n1(cc(nc1)c2cnccc2)CCC | 0.0003 |