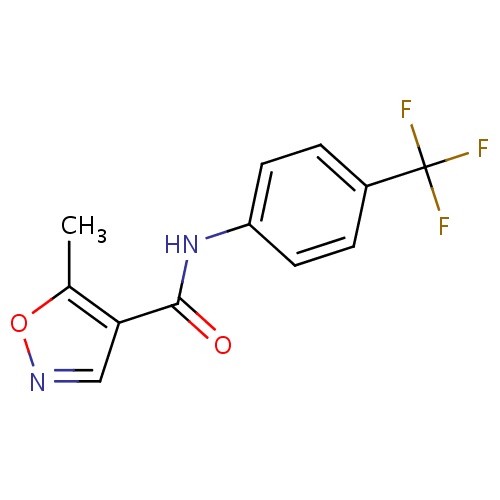
IUPAC name
5-methyl-N-[4-(trifluoromethyl)phenyl]-1,2-oxazole-4-carboxamide
SMILES
CC1=C(C=NO1)C(=O)NC1=CC=C(C=C1)C(F)(F)F
Compound class
Antirheumatic Agents; Immunosuppressive Agents; Enzyme Inhibitors; Adjuvants; Antineoplastic and Immunomodulating Agents; Selective Immunosuppressants; Cytochrome P-450 CYP1A2 Inhibitors; Cytochrome P-450 CYP2C9 Inhibitors; Cytochrome P-450 CYP1A2 Inducers; Cytochrome P-450 CYP2C9 Inducers;
Therapeutic area
For the management of the signs and symptoms of active rheumatoid arthritis (RA) to improve physical function and to slow the progression of structural damage associated with the disease. Has also been used for the prevention of acute and chronic rejection in recipients of solid organ trasnplants and is designated by the FDA as an orphan drug for this use.
Common name
Leflunomide
IUPAC name
5-methyl-N-[4-(trifluoromethyl)phenyl]-1,2-oxazole-4-carboxamide
SMILES
CC1=C(C=NO1)C(=O)NC1=CC=C(C=C1)C(F)(F)F
INCHI
InChI=1S/C12H9F3N2O2/c1-7-10(6-16-19-7)11(18)17-9-4-2-8(3-5-9)12(13,14)15/h2-6H,1H3,(H,17,18)
FORMULA
C12H9F3N2O2

Common name
Leflunomide
IUPAC name
5-methyl-N-[4-(trifluoromethyl)phenyl]-1,2-oxazole-4-carboxamide
Molecular weight
270.207
clogP
2.973
clogS
-4.274
HBond Acceptor
3
HBond Donor
1
Total Polar Surface Area
55.13
Number of Rings
2
Rotatable Bond
3
| Drug ID | Common name | Structure CAS | SMILE | Frequency |
|---|---|---|---|---|
| FDBF00003 | formamide |
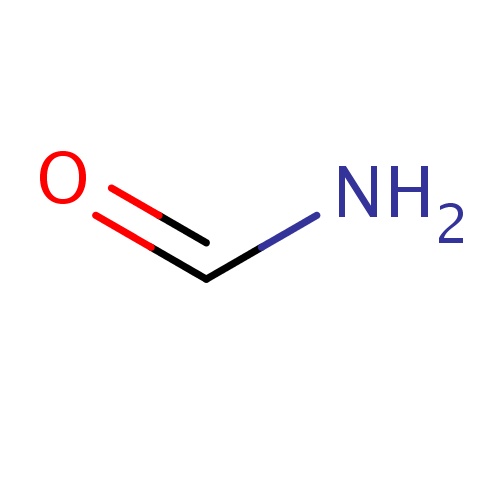
|
C(=O)N | 0.1240 |
| FDBF00005 | benzene |
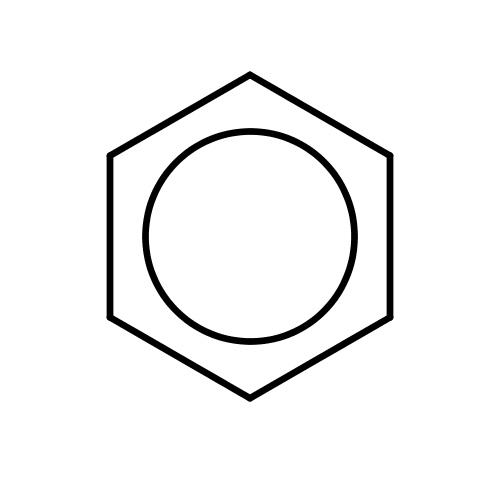
|
c1ccccc1 | 0.2824 |
| FDBF00162 | trifluoromethylbenzene |
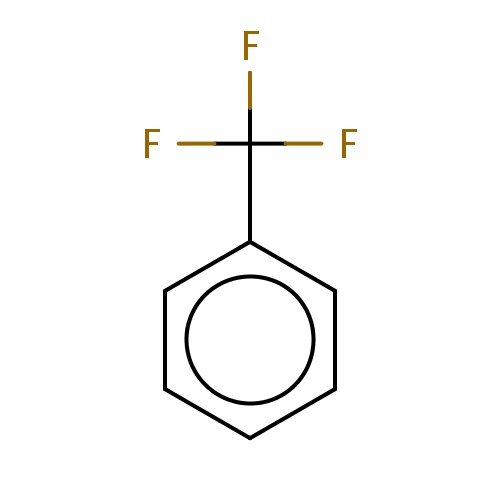
|
c1ccc(cc1)C(F)(F)F | 0.0172 |
| FDBF00177 | fluoroform |
|
FC(F)F | 0.0704 |
| FDBF00686 | 5-methylisoxazole |
|
o1nccc1C | 0.0031 |
| FDBF02490 | N-[4-(trifluoromethyl)phenyl]formamide |

|
c1(ccc(cc1)C(F)(F)F)NC=O | 0.0003 |
| FDBF02491 | 5-methyl-N-phenyl-isoxazole-4-carboxamide |

|
c1(ccccc1)NC(=O)c2cnoc2C | 0.0003 |

