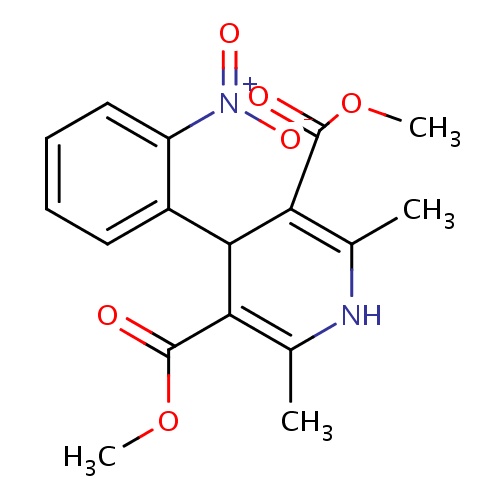
IUPAC name
3,5-dimethyl 2,6-dimethyl-4-(2-nitrophenyl)-1,4-dihydropyridine-3,5-dicarboxylate
SMILES
COC(=O)C1=C(C)NC(C)=C(C1C1=CC=CC=C1[N+]([O-])=O)C(=O)OC
Compound class
Vasodilator Agents; Calcium Channel Blockers; Tocolytic Agents; Dihydropyridines; Cardiovascular System; Dihydropyridine Derivatives; Selective Calcium Channel Blockers With Mainly Vascular Effects; Calcium Channel Blockers and Diuretics; Cytochrome P-450 CYP1A2 Inhibitors; Cytochrome P-450 CYP2C9 Inhibitors; Cytochrome P-450 CYP1A2 Inducers; Cytochrome P-450 CYP2C8 Inhibitors; Cytochrome P-450 CYP2C9 Inducers; Cytochrome P-450 CYP2C8 Inducers; Cytochrome P-450 CYP2B6 Inducers; Cytochrome P-450 CYP2B6 Inhibitors; CYP2A6 Inhibitors; CYP2A6 Inhibitors (strong); CYP2A6 Inhibitors (moderate); CYP2A6 Inducers; CYP2A6 Inducers (strong); CYP2B6 Inhibitors (strong); CYP2D6 Inducers; CYP2D6 Inducers (strong); CYP2E1 Inhibitors; CYP2E1 Inducers; CYP2E1 Inducers (strong); CYP3A4 Inhibitors; Combined Inducers of CYP3A4 and P-glycoprotein; Combined Inhibitors of CYP3A4 and P-glycoprotein;
Therapeutic area
For the management of vasospastic angina, chronic stable angina, hypertension, and Raynaud's phenomenon. May be used as a first line agent for left ventricular hypertrophy and isolated systolic hypertension (long-acting agents).
Common name
Nifedipine
IUPAC name
3,5-dimethyl 2,6-dimethyl-4-(2-nitrophenyl)-1,4-dihydropyridine-3,5-dicarboxylate
SMILES
COC(=O)C1=C(C)NC(C)=C(C1C1=CC=CC=C1[N+]([O-])=O)C(=O)OC
INCHI
InChI=1S/C17H18N2O6/c1-9-13(16(20)24-3)15(14(10(2)18-9)17(21)25-4)11-7-5-6-8-12(11)19(22)23/h5-8,15,18H,1-4H3
FORMULA
C17H18N2O6

Common name
Nifedipine
IUPAC name
3,5-dimethyl 2,6-dimethyl-4-(2-nitrophenyl)-1,4-dihydropyridine-3,5-dicarboxylate
Molecular weight
346.335
clogP
1.614
clogS
-3.839
HBond Acceptor
6
HBond Donor
1
Total Polar Surface Area
116.44
Number of Rings
2
Rotatable Bond
6
| Drug ID | Common name | Structure CAS | SMILE | Frequency |
|---|---|---|---|---|
| FDBF00779 | nitrobenzene |
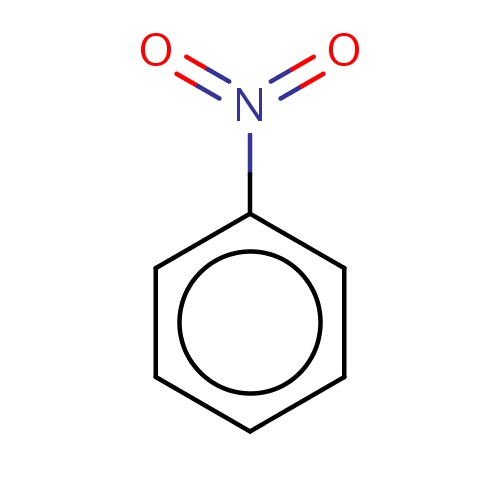
|
O=N(=O)c1ccccc1 | 0.0134 |

