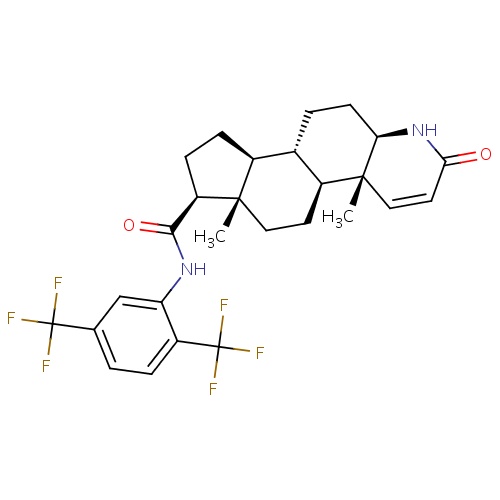
IUPAC name
(1S,2R,7R,10S,11S,14S,15S)-N-[2,5-bis(trifluoromethyl)phenyl]-2,15-dimethyl-5-oxo-6-azatetracyclo[8.7.0.0²,
SMILES
[H][C@@]1(CC[C@@]2([H])[C@]3([H])CC[C@@]4([H])NC(=O)C=C[C@]4(C)[C@@]3([H])CC[C@]12C)C(=O)NC1=CC(=CC=C1C(F)(F)F)C(F)(F)F
Compound class
Adrenergic alpha-Antagonists; 5-alpha Reductase Inhibitors; Genito Urinary System and Sex Hormones; Drugs Used in Benign Prostatic Hypertrophy; Testosterone-5-Alpha Reductase Inhibitors; Urological Agents; CYP3A4 Inhibitors;
Therapeutic area
For the treatment of symptomatic benign prostatic hyperplasia (BPH) in men with an enlarged prostate gland to improve symptoms, and reduce the risk of acute urinary retention and the need for surgery.
Common name
Dutasteride
IUPAC name
(1S,2R,7R,10S,11S,14S,15S)-N-[2,5-bis(trifluoromethyl)phenyl]-2,15-dimethyl-5-oxo-6-azatetracyclo[8.7.0.0²,
SMILES
[H][C@@]1(CC[C@@]2([H])[C@]3([H])CC[C@@]4([H])NC(=O)C=C[C@]4(C)[C@@]3([H])CC[C@]12C)C(=O)NC1=CC(=CC=C1C(F)(F)F)C(F)(F)F
INCHI
InChI=1S/C27H30F6N2O2/c1-24-11-9-17-15(4-8-21-25(17,2)12-10-22(36)35-21)16(24)6-7-19(24)23(37)34-20-13-14(26(28,29)30)3-5-18(20)27(31,32)33/h3,5,10,12-13,15-17,19,21H,4,6-9,11H2,1-2H3,(H,34,37)(H,35,36)/t15-,16-,17-,19+,21+,24-,25+/m0/s1
FORMULA
C27H30F6N2O2

Common name
Dutasteride
IUPAC name
(1S,2R,7R,10S,11S,14S,15S)-N-[2,5-bis(trifluoromethyl)phenyl]-2,15-dimethyl-5-oxo-6-azatetracyclo[8.7.0.0²,
Molecular weight
528.530
clogP
5.590
clogS
-7.012
HBond Acceptor
2
HBond Donor
2
Total Polar Surface Area
58.2
Number of Rings
5
Rotatable Bond
4
| Drug ID | Common name | Structure CAS | SMILE | Frequency |
|---|---|---|---|---|
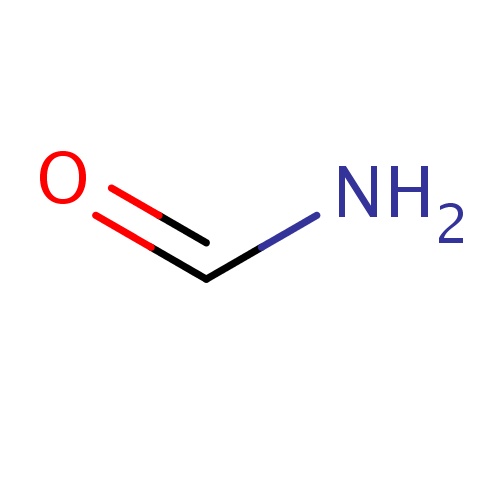
| FDBF00003 | formamide |
|
C(=O)N | 0.1240 |
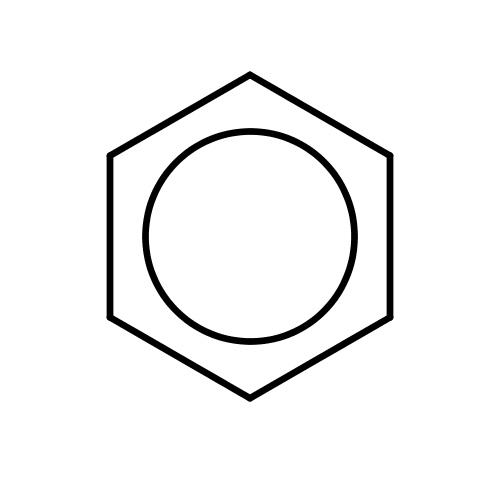
| FDBF00005 | benzene |
|
c1ccccc1 | 0.2824 |
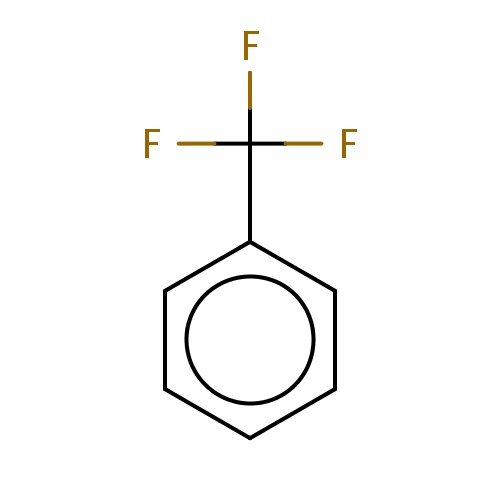
| FDBF00162 | trifluoromethylbenzene |
|
c1ccc(cc1)C(F)(F)F | 0.0172 |
| FDBF00177 | fluoroform |
|
FC(F)F | 0.0704 |
| FDBF02548 | N-[2-(trifluoromethyl)phenyl]formamide |

|
c1(c(cccc1)C(F)(F)F)NC=O | 0.0003 |
| FDBF02549 | N-[3-(trifluoromethyl)phenyl]formamide |
|
c1(cccc(c1)C(F)(F)F)NC=O | 0.0010 |

