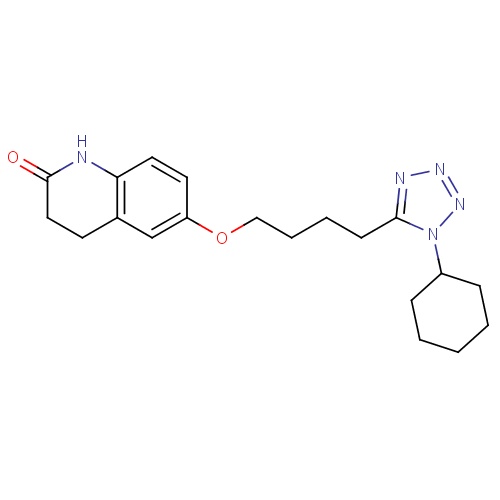
IUPAC name
6-[4-(1-cyclohexyl-1H-1,2,3,4-tetrazol-5-yl)butoxy]-1,2,3,4-tetrahydroquinolin-2-one
SMILES
O=C1CCC2=C(N1)C=CC(OCCCCC1=NN=NN1C1CCCCC1)=C2
Compound class
Fibrinolytic Agents; Platelet Aggregation Inhibitors; Bronchodilator Agents; Phosphodiesterase 3 Inhibitors; Vasodilator Agents; Neuroprotective Agents; Antithrombotic Agents; Blood and Blood Forming Organs; Platelet Aggregation Inhibitors Excl. Heparin; Cytochrome P-450 CYP1A2 Inhibitors; Cytochrome P-450 CYP1A2 Inducers; Cytochrome P-450 CYP2C19 Inducers; CYP2D6 Inducers; CYP2D6 Inducers (strong); CYP3A4 Inhibitors;
Therapeutic area
For the reduction of symptoms of intermittent claudication (pain in the legs that occurs with walking and disappears with rest).
Common name
Cilostazol
IUPAC name
6-[4-(1-cyclohexyl-1H-1,2,3,4-tetrazol-5-yl)butoxy]-1,2,3,4-tetrahydroquinolin-2-one
SMILES
O=C1CCC2=C(N1)C=CC(OCCCCC1=NN=NN1C1CCCCC1)=C2
INCHI
InChI=1S/C20H27N5O2/c26-20-12-9-15-14-17(10-11-18(15)21-20)27-13-5-4-8-19-22-23-24-25(19)16-6-2-1-3-7-16/h10-11,14,16H,1-9,12-13H2,(H,21,26)
FORMULA
C20H27N5O2

Common name
Cilostazol
IUPAC name
6-[4-(1-cyclohexyl-1H-1,2,3,4-tetrazol-5-yl)butoxy]-1,2,3,4-tetrahydroquinolin-2-one
Molecular weight
369.461
clogP
3.101
clogS
-5.241
HBond Acceptor
5
HBond Donor
1
Total Polar Surface Area
81.93
Number of Rings
4
Rotatable Bond
7
| Drug ID | Common name | Structure CAS | SMILE | Frequency |
|---|---|---|---|---|
| FDBF00007 | propane |

|
C(C)C | 0.2412 |
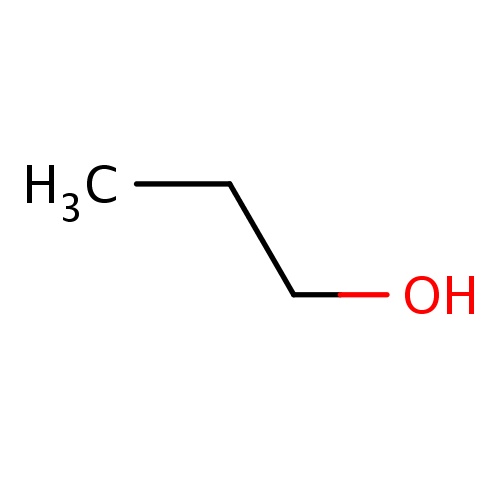
| FDBF00018 | propan-1-ol |
|
C(O)CC | 0.0330 |
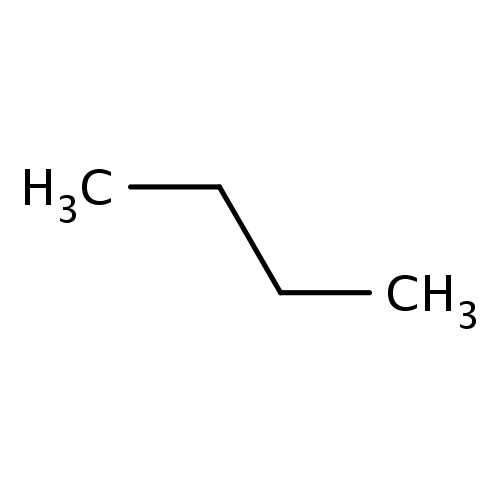
| FDBF00067 | butane |
|
CCCC | 0.0680 |
| FDBF00355 | cyclohexane |

|
C1CCCCC1 | 0.0127 |
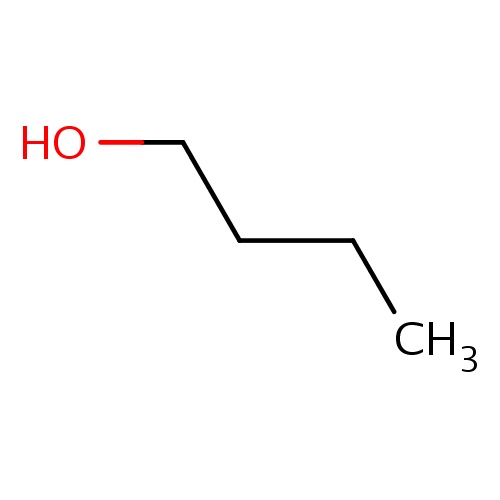
| FDBF00416 | butan-1-ol |
|
C(CC)CO | 0.0134 |
| FDBF02631 | 6-hydroxy-3,4-dihydro-1H-quinolin-2-one |

|
Oc1cc2c(cc1)NC(=O)CC2 | 0.0003 |
| FDBF02632 | 1-cyclohexyltetrazole |

|
n1(nnnc1)C2CCCCC2 | 0.0003 |
| FDBF02635 | 1-cyclohexyl-5-methyl-tetrazole |

|
n1(nnnc1C)C2CCCCC2 | 0.0003 |
| FDBF02638 | 5-propyl-1H-tetrazole |

|
[nH]1nnnc1CCC | 0.0003 |
| FDBF02642 | 6-propoxy-3,4-dihydro-1H-quinolin-2-one |
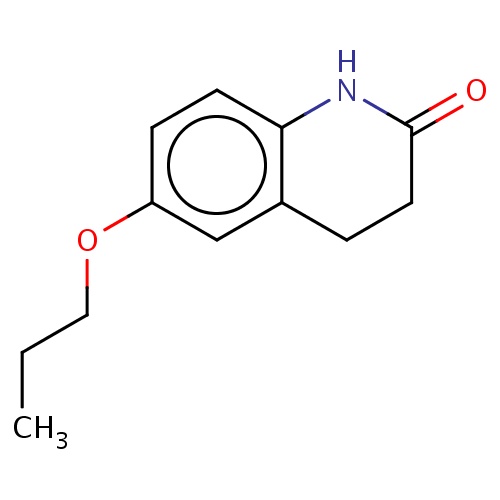
|
O(c1cc2c(cc1)NC(=O)CC2)CCC | 0.0003 |

