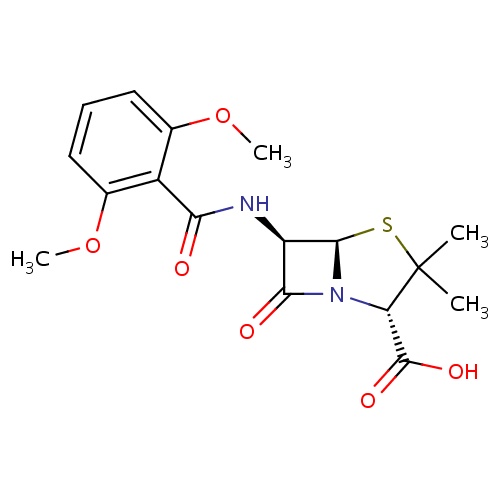
IUPAC name
(2S,5R,6R)-6-(2,6-dimethoxybenzamido)-3,3-dimethyl-7-oxo-4-thia-1-azabicyclo[3.2.0]heptane-2-carboxylic acid
SMILES
[H][C@]12SC(C)(C)[C@@H](N1C(=O)[C@H]2NC(=O)C1=C(OC)C=CC=C1OC)C(O)=O
Compound class
Anti-Bacterial Agents; Penicillins; Antibacterials for Systemic Use; Antiinfectives for Systemic Use; Antibacterials for Intramammary Use; Beta-Lactamase Resistant Penicillins; Beta-Lactam Antibacterials, Penicillins; Beta-Lactam Antibacterials, Penicillins, for Intramammary Use;
Therapeutic area
Used to treat infections caused by susceptible Gram-positive bacteria, particularly beta-lactamase-producing organisms such as .
Common name
Meticillin
IUPAC name
(2S,5R,6R)-6-(2,6-dimethoxybenzamido)-3,3-dimethyl-7-oxo-4-thia-1-azabicyclo[3.2.0]heptane-2-carboxylic acid
SMILES
[H][C@]12SC(C)(C)[C@@H](N1C(=O)[C@H]2NC(=O)C1=C(OC)C=CC=C1OC)C(O)=O
INCHI
InChI=1S/C17H20N2O6S/c1-17(2)12(16(22)23)19-14(21)11(15(19)26-17)18-13(20)10-8(24-3)6-5-7-9(10)25-4/h5-7,11-12,15H,1-4H3,(H,18,20)(H,22,23)/t11-,12+,15-/m1/s1
FORMULA
C17H20N2O6S

Common name
Meticillin
IUPAC name
(2S,5R,6R)-6-(2,6-dimethoxybenzamido)-3,3-dimethyl-7-oxo-4-thia-1-azabicyclo[3.2.0]heptane-2-carboxylic acid
Molecular weight
380.415
clogP
0.777
clogS
-2.300
HBond Acceptor
6
HBond Donor
2
Total Polar Surface Area
130.47
Number of Rings
3
Rotatable Bond
5
| Drug ID | Common name | Structure CAS | SMILE | Frequency |
|---|---|---|---|---|
| FDBF00003 | formamide |
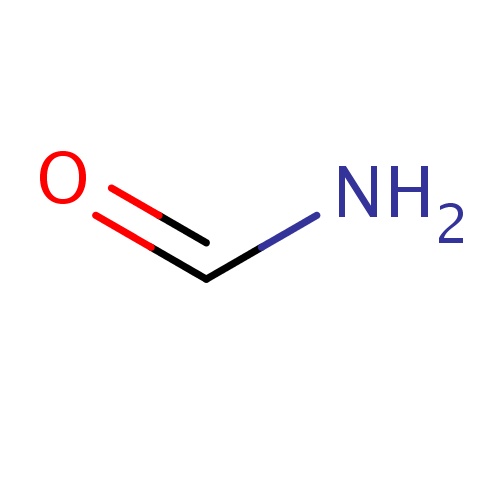
|
C(=O)N | 0.1240 |
| FDBF00005 | benzene |
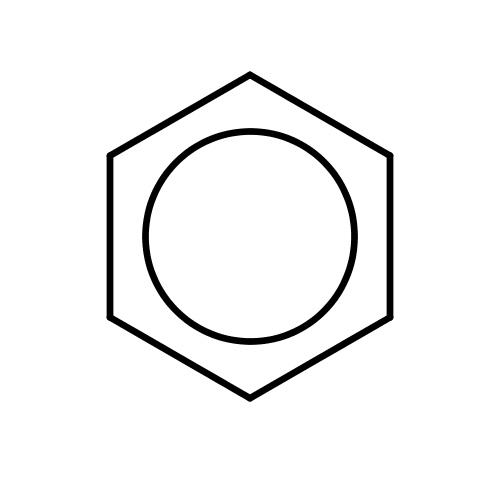
|
c1ccccc1 | 0.2824 |
| FDBF00048 | benzamide |
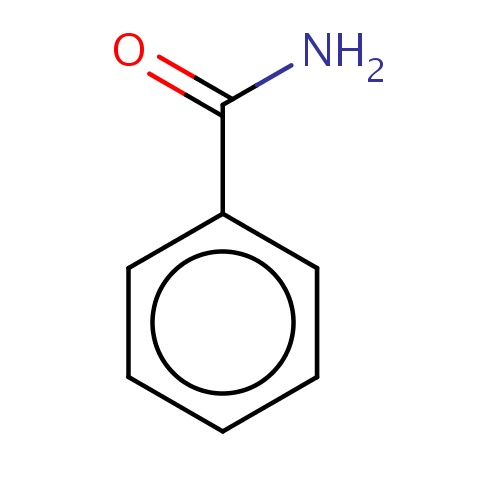
|
O=C(N)c1ccccc1 | 0.0117 |
| FDBF00211 | anisole |
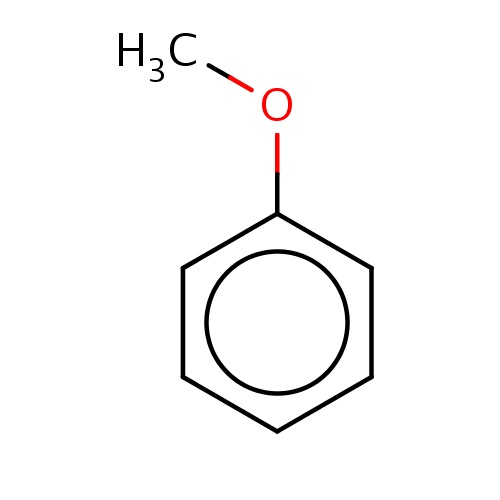
|
COc1ccccc1 | 0.0423 |
| FDBF00296 | 1,3-dimethoxybenzene |
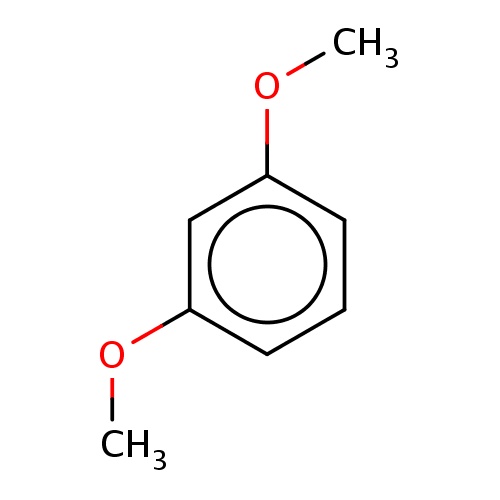
|
c1cc(cc(c1)OC)OC | 0.0041 |
| FDBF00859 | 2-methoxybenzamide |
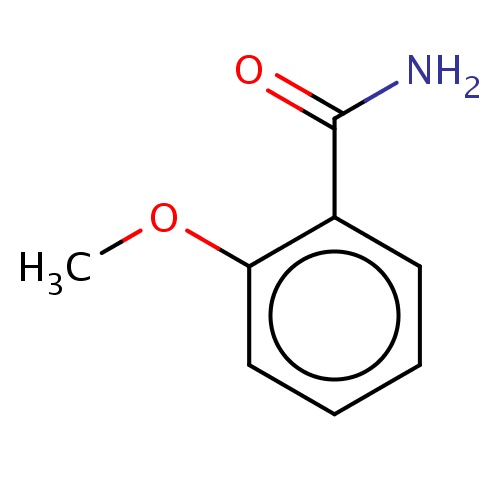
|
c1(ccccc1OC)C(=O)N | 0.0014 |

