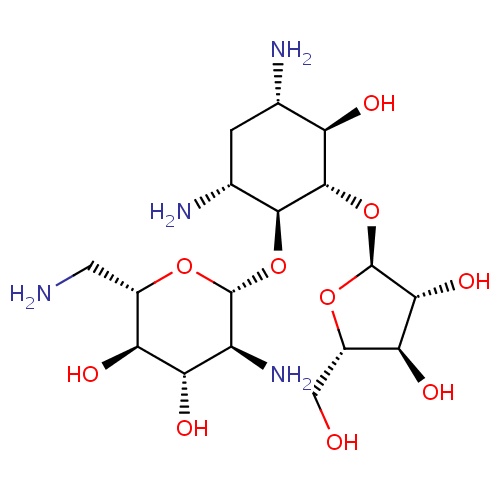
IUPAC name
(2S,3R,4S,5S,6R)-5-amino-2-(aminomethyl)-6-{[(1S,2S,3R,4S,6R)-4,6-diamino-2-{[(2S,3R,4R,5S)-3,4-dihydroxy-5-(hydroxymethyl)oxolan-2-yl]oxy}-3-hydroxycyclohexyl]oxy}oxane-3,4-diol
SMILES
NC[C@@H]1O[C@H](O[C@H]2[C@H](N)C[C@H](N)[C@@H](O)[C@@H]2O[C@@H]2O[C@@H](CO)[C@H](O)[C@H]2O)[C@@H](N)[C@H](O)[C@H]1O
Compound class
Anti-Bacterial Agents; Aminoglycosides; Antibacterials for Systemic Use; Antiinfectives for Systemic Use; Aminoglycoside Antibacterials;
Therapeutic area
Common name
Ribostamycin
IUPAC name
(2S,3R,4S,5S,6R)-5-amino-2-(aminomethyl)-6-{[(1S,2S,3R,4S,6R)-4,6-diamino-2-{[(2S,3R,4R,5S)-3,4-dihydroxy-5-(hydroxymethyl)oxolan-2-yl]oxy}-3-hydroxycyclohexyl]oxy}oxane-3,4-diol
SMILES
NC[C@@H]1O[C@H](O[C@H]2[C@H](N)C[C@H](N)[C@@H](O)[C@@H]2O[C@@H]2O[C@@H](CO)[C@H](O)[C@H]2O)[C@@H](N)[C@H](O)[C@H]1O
INCHI
InChI=1S/C17H34N4O10/c18-2-6-10(24)12(26)8(21)16(28-6)30-14-5(20)1-4(19)9(23)15(14)31-17-13(27)11(25)7(3-22)29-17/h4-17,22-27H,1-3,18-21H2/t4-,5+,6-,7-,8-,9+,10-,11-,12-,13+,14-,15-,16+,17-/m0/s1
FORMULA
C17H34N4O10

Common name
Ribostamycin
IUPAC name
(2S,3R,4S,5S,6R)-5-amino-2-(aminomethyl)-6-{[(1S,2S,3R,4S,6R)-4,6-diamino-2-{[(2S,3R,4R,5S)-3,4-dihydroxy-5-(hydroxymethyl)oxolan-2-yl]oxy}-3-hydroxycyclohexyl]oxy}oxane-3,4-diol
Molecular weight
454.473
clogP
-6.569
clogS
4.425
HBond Acceptor
10
HBond Donor
14
Total Polar Surface Area
262.38
Number of Rings
3
Rotatable Bond
6
| Drug ID | Common name | Structure CAS | SMILE | Frequency |
|---|---|---|---|---|
| FDBF01457 | (3S,4S)-tetrahydrofuran-3,4-diol |

|
O1CC(C(C1)O)O | 0.0010 |

