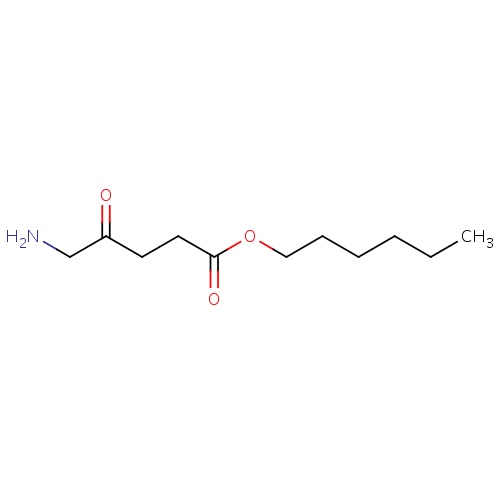
IUPAC name
hexyl 5-amino-4-oxopentanoate
SMILES
[H]N([H])CC(=O)CCC(=O)OCCCCCC
Compound class
;
Therapeutic area
Hexaminolevulinate is indicated for use in the cystoscopic detection of non-muscle invasive papillary cancer of the bladder among patients suspected or known to have lesion(s) on the basis of a prior cystoscopy.
Common name
Hexaminolevulinate
IUPAC name
hexyl 5-amino-4-oxopentanoate
SMILES
[H]N([H])CC(=O)CCC(=O)OCCCCCC
INCHI
InChI=1S/C11H21NO3/c1-2-3-4-5-8-15-11(14)7-6-10(13)9-12/h2-9,12H2,1H3
FORMULA
C11H21NO3

Common name
Hexaminolevulinate
IUPAC name
hexyl 5-amino-4-oxopentanoate
Molecular weight
215.289
clogP
2.042
clogS
-2.792
HBond Acceptor
3
HBond Donor
2
Total Polar Surface Area
69.39
Number of Rings
0
Rotatable Bond
10
| Drug ID | Common name | Structure CAS | SMILE | Frequency |
|---|---|---|---|---|
| FDBF00007 | propane |

|
C(C)C | 0.2412 |
| FDBF00098 | acetaldehyde |
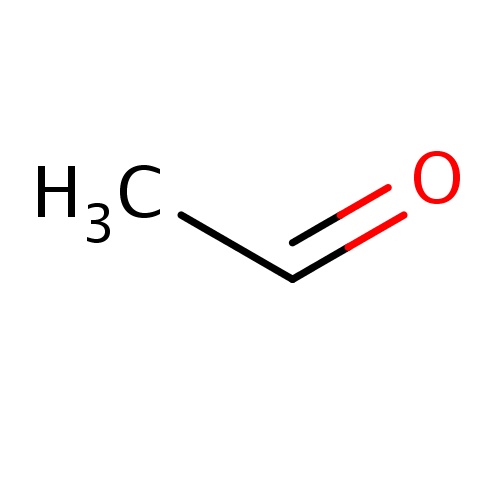
|
CC=O | 0.0182 |
| FDBF00100 | ethyl formate |

|
O(C=O)CC | 0.0244 |
| FDBF00101 | methyl acetate |
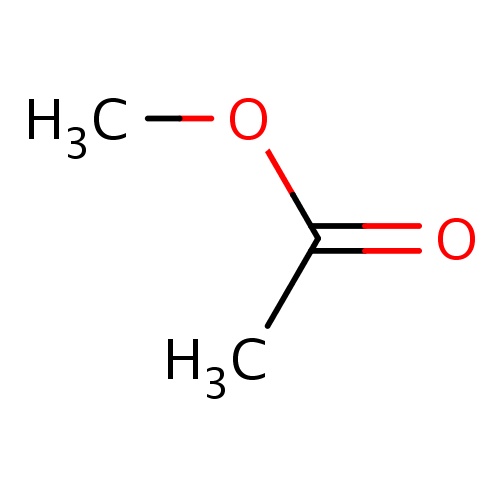
|
O(C(=O)C)C | 0.0151 |
| FDBF00105 | methyl formate |
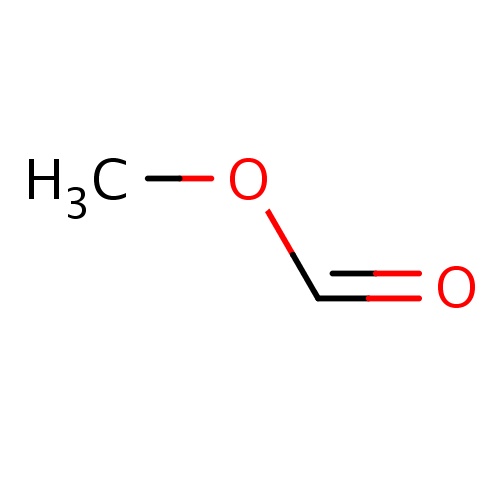
|
O(C=O)C | 0.0323 |
| FDBF00190 | ethyl propanoate |
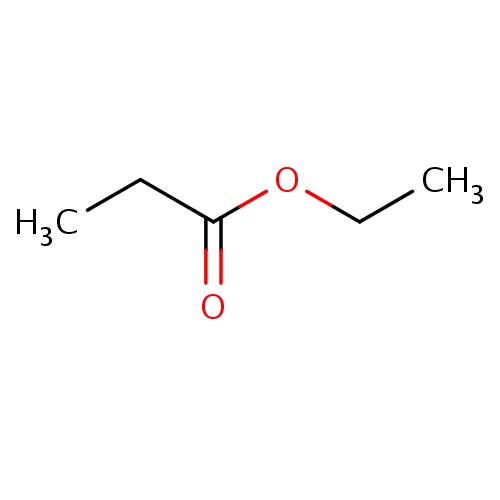
|
C(C)C(=O)OCC | 0.0086 |
| FDBF00927 | propyl acetate |

|
C(COC(=O)C)C | 0.0017 |
| FDBF01477 | propyl formate |
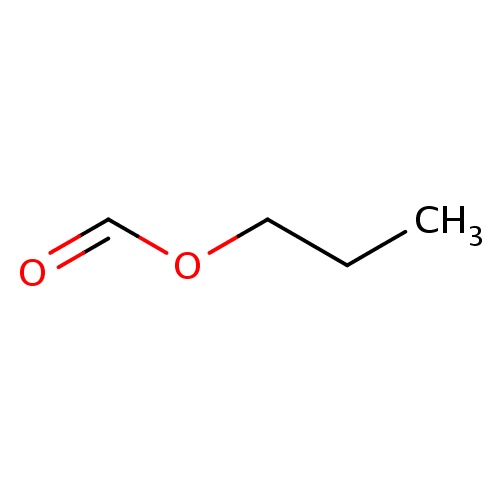
|
O(C=O)CCC | 0.0021 |
| FDBF01910 | 2-aminoacetaldehyde |

|
O=CCN | 0.0010 |
| FDBF01912 | 1-aminopropan-2-one |
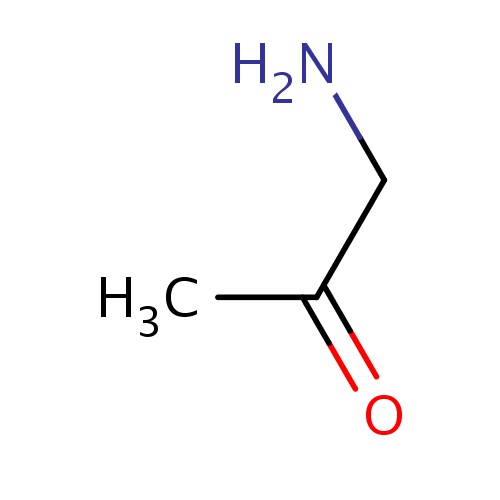
|
O=C(CN)C | 0.0010 |
16 ,
2

