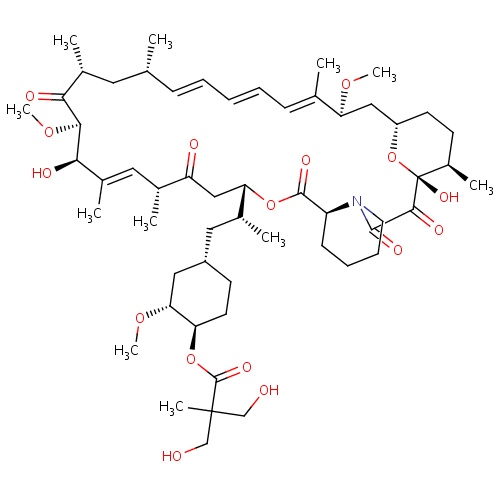
IUPAC name
(1R,2R,4S)-4-[(2R)-2-[(1R,9S,12S,15R,16E,18R,19R,21R,23S,24E,26E,28E,30S,32S,35R)-1,18-dihydroxy-19,30-dimethoxy-15,17,21,23,29,35-hexamethyl-2,3,10,14,20-pentaoxo-11,36-dioxa-4-azatricyclo[30.3.1.0
SMILES
OCC(C)(CO)C(=O)O[C@@H]1CC[C@@H](C[C@@H](C)[C@]2([H])CC(=O)[C@H](C)\C=C(C)\[C@@H](O)[C@@H](OC)C(=O)[C@H](C)C[C@H](C)\C=C\C=C\C=C(C)\[C@@H](OC)C[C@]3([H])CC[C@@H](C)[C@@](O)(O3)C(=O)C(=O)N3CCCC[C@H]3C(=O)O2)C[C@H]1OC
Compound class
Antineoplastic Agents; Immunosuppressive Agents; Protein Kinase Inhibitors; Antineoplastic and Immunomodulating Agents; CYP2D6 Inducers; CYP2D6 Inducers (strong); CYP3A4 Inhibitors; Combined Inhibitors of CYP3A4 and P-glycoprotein;
Therapeutic area
For the treatment of renal cell carcinoma (RCC). Also investigated for use/treatment in breast cancer, lymphoma (unspecified), rheumatoid arthritis, and multiple myeloma.
Common name
Temsirolimus
IUPAC name
(1R,2R,4S)-4-[(2R)-2-[(1R,9S,12S,15R,16E,18R,19R,21R,23S,24E,26E,28E,30S,32S,35R)-1,18-dihydroxy-19,30-dimethoxy-15,17,21,23,29,35-hexamethyl-2,3,10,14,20-pentaoxo-11,36-dioxa-4-azatricyclo[30.3.1.0
SMILES
OCC(C)(CO)C(=O)O[C@@H]1CC[C@@H](C[C@@H](C)[C@]2([H])CC(=O)[C@H](C)\C=C(C)\[C@@H](O)[C@@H](OC)C(=O)[C@H](C)C[C@H](C)\C=C\C=C\C=C(C)\[C@@H](OC)C[C@]3([H])CC[C@@H](C)[C@@](O)(O3)C(=O)C(=O)N3CCCC[C@H]3C(=O)O2)C[C@H]1OC
INCHI
InChI=1S/C56H87NO16/c1-33-17-13-12-14-18-34(2)45(68-9)29-41-22-20-39(7)56(67,73-41)51(63)52(64)57-24-16-15-19-42(57)53(65)71-46(30-43(60)35(3)26-38(6)49(62)50(70-11)48(61)37(5)25-33)36(4)27-40-21-23-44(47(28-40)69-10)72-54(66)55(8,31-58)32-59/h12-14,17-18,26,33,35-37,39-42,44-47,49-50,58-59,62,67H,15-16,19-25,27-32H2,1-11H3/b14-12+,17-13+,34-18+,38-26+/t33-,35-,36-,37-,39-,40+,41+,42+,44-,45+,46+,47-,49-,50+,56-/m1/s1
FORMULA
C56H87NO16

Common name
Temsirolimus
IUPAC name
(1R,2R,4S)-4-[(2R)-2-[(1R,9S,12S,15R,16E,18R,19R,21R,23S,24E,26E,28E,30S,32S,35R)-1,18-dihydroxy-19,30-dimethoxy-15,17,21,23,29,35-hexamethyl-2,3,10,14,20-pentaoxo-11,36-dioxa-4-azatricyclo[30.3.1.0
Molecular weight
1030.287
clogP
3.976
clogS
-5.736
HBond Acceptor
16
HBond Donor
4
Total Polar Surface Area
241.96
Number of Rings
4
Rotatable Bond
11
| Drug ID | Common name | Structure CAS | SMILE | Frequency |
|---|---|---|---|---|
| FDBF00007 | propane |

|
C(C)C | 0.2412 |
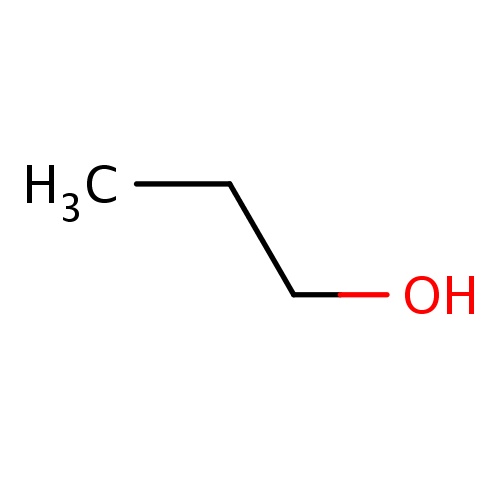
| FDBF00018 | propan-1-ol |
|
C(O)CC | 0.0330 |
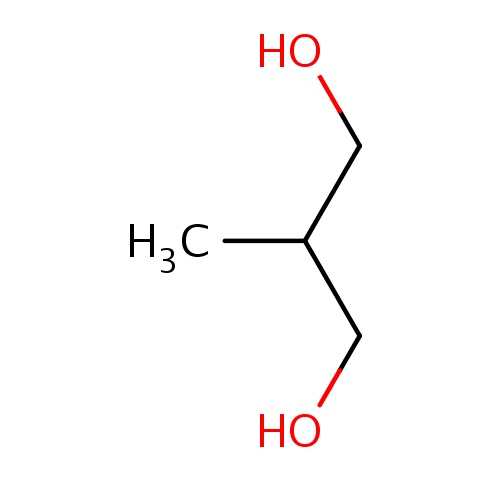
| FDBF00681 | 2-methylpropane-1,3-diol |
|
C(O)C(CO)C | 0.0007 |
| FDBF03441 | (4-methylcyclohexyl) formate |

|
C1(CCC(CC1)OC=O)C | 0.0003 |
| FDBF03442 | [(1R,2R)-2-methoxycyclohexyl] formate |

|
O(C)C1CCCCC1OC=O | 0.0003 |
| FDBF03443 | cyclohexyl propanoate |

|
C(C)C(=O)OC1CCCCC1 | 0.0003 |
| FDBF03444 | [(1R,2R,4S)-2-methoxy-4-methyl-cyclohexyl] formate |

|
O(C)C1CC(CCC1OC=O)C | 0.0003 |
| FDBF03445 | (4-methylcyclohexyl) propanoate |

|
C(C)C(=O)OC1CCC(CC1)C | 0.0003 |
| FDBF03446 | cyclohexyl formate |

|
C1CCC(CC1)OC=O | 0.0003 |

