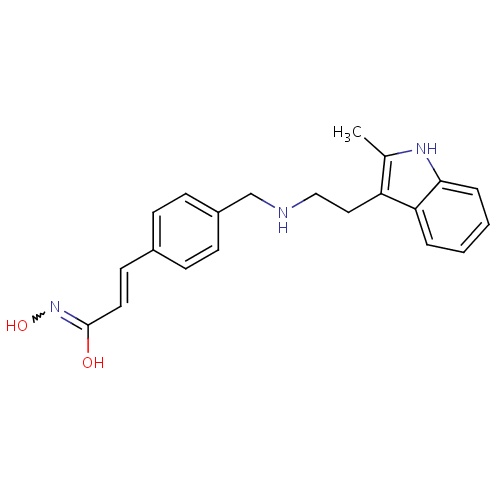
IUPAC name
N-hydroxy(2E)-3-[4-({[2-(2-methyl-1H-indol-3-yl)ethyl]amino}methyl)phenyl]prop-2-enimidic acid
SMILES
[H]\C(=C(\[H])C1=CC=C(CNCCC2=C(C)NC3=CC=CC=C23)C=C1)C(O)=NO
Compound class
Antineoplastic Agents; Immunosuppressive Agents; Histone Deacetylase Inhibitors; Antineoplastic and Immunomodulating Agents; CYP2D6 Inducers; CYP2D6 Inducers (strong); CYP3A4 Inhibitors;
Therapeutic area
Panobinostat is indicated in the treatment of multiple myeloma in combination with dexamethasone and bortezomib in patients who have received 2 previous treatment regimens including bortezomib and an immunomodulatory agent. This indication is approved by accelerated approval based on progression free survival as of February 23,2015
Common name
Panobinostat
IUPAC name
N-hydroxy(2E)-3-[4-({[2-(2-methyl-1H-indol-3-yl)ethyl]amino}methyl)phenyl]prop-2-enimidic acid
SMILES
[H]\C(=C(\[H])C1=CC=C(CNCCC2=C(C)NC3=CC=CC=C23)C=C1)C(O)=NO
INCHI
InChI=1S/C21H23N3O2/c1-15-18(19-4-2-3-5-20(19)23-15)12-13-22-14-17-8-6-16(7-9-17)10-11-21(25)24-26/h2-11,22-23,26H,12-14H2,1H3,(H,24,25)/b11-10+
FORMULA
C21H23N3O2

Common name
Panobinostat
IUPAC name
N-hydroxy(2E)-3-[4-({[2-(2-methyl-1H-indol-3-yl)ethyl]amino}methyl)phenyl]prop-2-enimidic acid
Molecular weight
349.426
clogP
4.621
clogS
-5.237
HBond Acceptor
3
HBond Donor
4
Total Polar Surface Area
80.64
Number of Rings
3
Rotatable Bond
7
| Drug ID | Common name | Structure CAS | SMILE | Frequency |
|---|---|---|---|---|
| FDBF00005 | benzene |
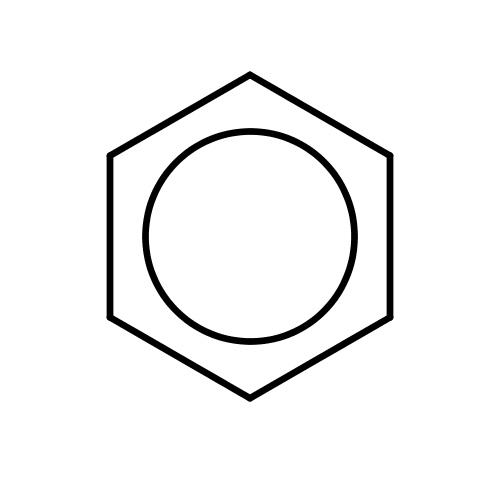
|
c1ccccc1 | 0.2824 |
| FDBF00023 | toluene |
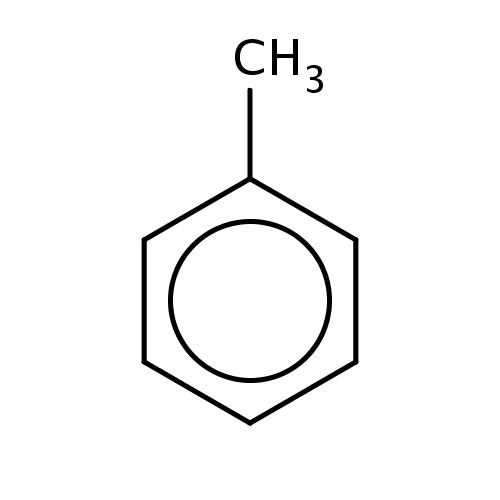
|
c1(ccccc1)C | 0.1268 |
| FDBF00040 | ethanamine |
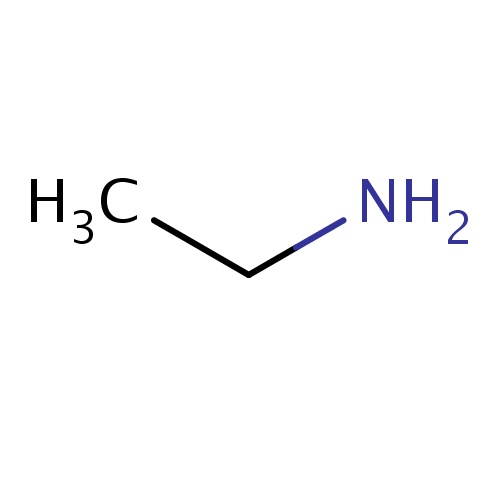
|
CCN | 0.0677 |
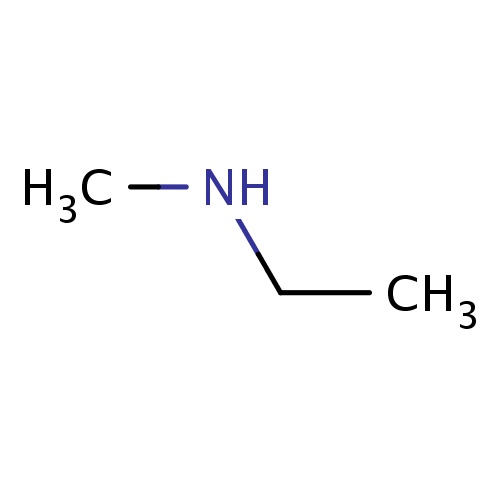
| FDBF00068 | N-methylethanamine |
|
N(CC)C | 0.0429 |
| FDBF00913 | phenylmethanamine |

|
NCc1ccccc1 | 0.0086 |
| FDBF01425 | N-methyl-1-phenyl-methanamine |

|
N(C)Cc1ccccc1 | 0.0045 |
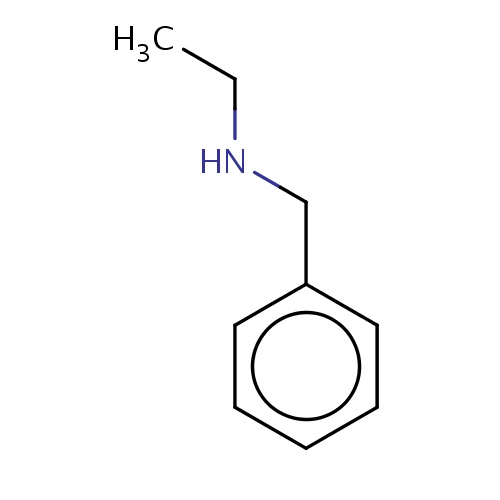
| FDBF01799 | N-benzylethanamine |
|
N(CC)Cc1ccccc1 | 0.0024 |
| FDBF03494 | (E)-3-phenylprop-2-enehydroxamic acid |

|
c1ccc(cc1)C=CC(=O)NO | 0.0003 |
| FDBF03495 | (E)-3-(p-tolyl)prop-2-enehydroxamic acid |

|
c1(ccc(cc1)C=CC(=O)NO)C | 0.0003 |

