IUPAC name
ethyl 3-(1-{2-[({4-[amino({[(hexyloxy)carbonyl]imino})methyl]phenyl}amino)methyl]-1-methyl-1H-1,3-benzodiazol-5-yl}-N-(pyridin-2-yl)formamido)propanoate
SMILES
CCCCCCOC(=O)N=C(N)C1=CC=C(NCC2=NC3=C(C=CC(=C3)C(=O)N(CCC(=O)OCC)C3=CC=CC=N3)N2C)C=C1
Compound class
Antithrombins; Direct Thrombin Inhibitors; Antithrombotic Agents; Blood and Blood Forming Organs;
Therapeutic area
Dabigatran is indicated for the prevention of venous thromboembolic events in patients who have undergone elective hip or knee replacement surgery (based on RE-NOVATE, RE-MODEL, and RE-MOBILIZE trials). In 2010, it was approved in the US and Canada for prevention of stroke and systemic embolism in patients with atrial fibrillation (approval based on the RE-LY trial). Contraindications: severe renal impairment (CrCL .
Common name
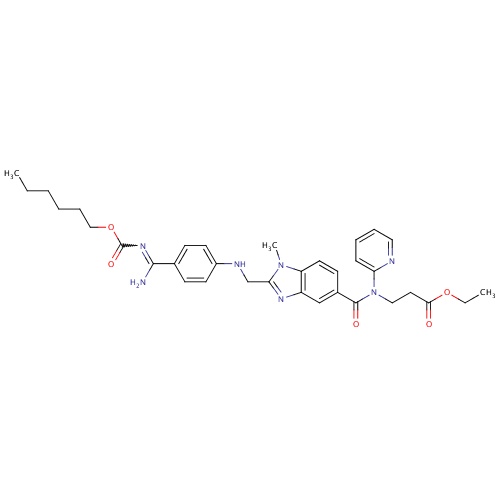
Dabigatran etexilate
IUPAC name
ethyl 3-(1-{2-[({4-[amino({[(hexyloxy)carbonyl]imino})methyl]phenyl}amino)methyl]-1-methyl-1H-1,3-benzodiazol-5-yl}-N-(pyridin-2-yl)formamido)propanoate
SMILES
CCCCCCOC(=O)N=C(N)C1=CC=C(NCC2=NC3=C(C=CC(=C3)C(=O)N(CCC(=O)OCC)C3=CC=CC=N3)N2C)C=C1
INCHI
InChI=1S/C34H41N7O5/c1-4-6-7-10-21-46-34(44)39-32(35)24-12-15-26(16-13-24)37-23-30-38-27-22-25(14-17-28(27)40(30)3)33(43)41(20-18-31(42)45-5-2)29-11-8-9-19-36-29/h8-9,11-17,19,22,37H,4-7,10,18,20-21,23H2,1-3H3,(H2,35,39,44)
FORMULA
C34H41N7O5

Common name
Dabigatran etexilate
IUPAC name
ethyl 3-(1-{2-[({4-[amino({[(hexyloxy)carbonyl]imino})methyl]phenyl}amino)methyl]-1-methyl-1H-1,3-benzodiazol-5-yl}-N-(pyridin-2-yl)formamido)propanoate
Molecular weight
627.733
clogP
4.869
clogS
-7.997
HBond Acceptor
7
HBond Donor
3
Total Polar Surface Area
154.03
Number of Rings
4
Rotatable Bond
19