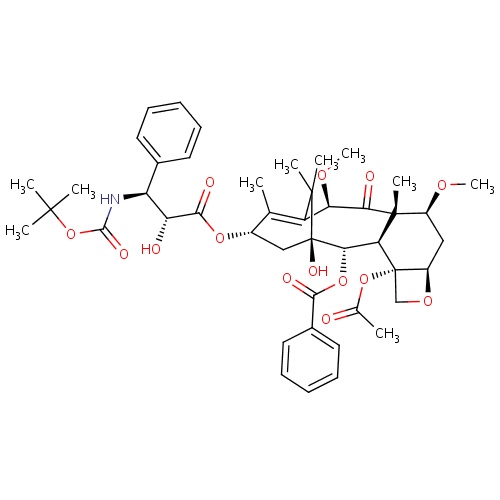
IUPAC name
(1S,2S,3R,4S,7R,9S,10S,12R,15S)-4-(acetyloxy)-15-{[(2R,3S)-3-{[(tert-butoxy)carbonyl]amino}-2-hydroxy-3-phenylpropanoyl]oxy}-1-hydroxy-9,12-dimethoxy-10,14,17,17-tetramethyl-11-oxo-6-oxatetracyclo[11.3.1.0³,¹
SMILES
[H][C@@](O)([C@@H](NC(=O)OC(C)(C)C)C1=CC=CC=C1)C(=O)O[C@@]1([H])C[C@@]2(O)[C@@]([H])(OC(=O)C3=CC=CC=C3)[C@]3([H])[C@@]4(CO[C@]4([H])C[C@]([H])(OC)[C@@]3(C)C(=O)[C@]([H])(OC)C(=C1C)C2(C)C)OC(C)=O
Compound class
Antineoplastic Agents; Immunosuppressive Agents; Antineoplastic and Immunomodulating Agents; Taxanes; Cytochrome P-450 CYP2C8 Inhibitors; Cytochrome P-450 CYP2C8 Inducers; CYP3A4 Inhibitors;
Therapeutic area
For treatment of patients with hormone-refractory metastatic prostate cancer previously treated with a docetaxel-containing treatment regimen.
Common name
Cabazitaxel
IUPAC name
(1S,2S,3R,4S,7R,9S,10S,12R,15S)-4-(acetyloxy)-15-{[(2R,3S)-3-{[(tert-butoxy)carbonyl]amino}-2-hydroxy-3-phenylpropanoyl]oxy}-1-hydroxy-9,12-dimethoxy-10,14,17,17-tetramethyl-11-oxo-6-oxatetracyclo[11.3.1.0³,¹
SMILES
[H][C@@](O)([C@@H](NC(=O)OC(C)(C)C)C1=CC=CC=C1)C(=O)O[C@@]1([H])C[C@@]2(O)[C@@]([H])(OC(=O)C3=CC=CC=C3)[C@]3([H])[C@@]4(CO[C@]4([H])C[C@]([H])(OC)[C@@]3(C)C(=O)[C@]([H])(OC)C(=C1C)C2(C)C)OC(C)=O
INCHI
InChI=1S/C45H57NO14/c1-24-28(57-39(51)33(48)32(26-17-13-11-14-18-26)46-40(52)60-41(3,4)5)22-45(53)37(58-38(50)27-19-15-12-16-20-27)35-43(8,36(49)34(55-10)31(24)42(45,6)7)29(54-9)21-30-44(35,23-56-30)59-25(2)47/h11-20,28-30,32-35,37,48,53H,21-23H2,1-10H3,(H,46,52)/t28-,29-,30+,32-,33+,34+,35-,37-,43+,44-,45+/m0/s1
FORMULA
C45H57NO14

Common name
Cabazitaxel
IUPAC name
(1S,2S,3R,4S,7R,9S,10S,12R,15S)-4-(acetyloxy)-15-{[(2R,3S)-3-{[(tert-butoxy)carbonyl]amino}-2-hydroxy-3-phenylpropanoyl]oxy}-1-hydroxy-9,12-dimethoxy-10,14,17,17-tetramethyl-11-oxo-6-oxatetracyclo[11.3.1.0³,¹
Molecular weight
835.932
clogP
4.658
clogS
-6.507
HBond Acceptor
14
HBond Donor
3
Total Polar Surface Area
202.45
Number of Rings
6
Rotatable Bond
15