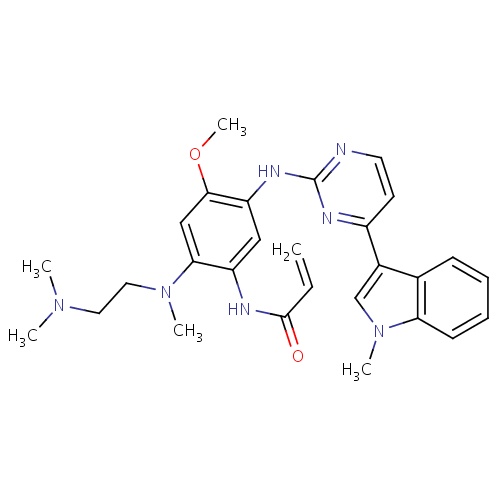
IUPAC name
N-(2-{[2-(dimethylamino)ethyl](methyl)amino}-4-methoxy-5-{[4-(1-methyl-1H-indol-3-yl)pyrimidin-2-yl]amino}phenyl)prop-2-enamide
SMILES
COC1=C(NC2=NC=CC(=N2)C2=CN(C)C3=C2C=CC=C3)C=C(NC(=O)C=C)C(=C1)N(C)CCN(C)C
Compound class
Antineoplastic Agents; Protein Kinase Inhibitors; Cytochrome P-450 CYP1A2 Inhibitors; Cytochrome P-450 CYP1A2 Inducers; CYP3A4 Inhibitors;
Therapeutic area
Osimertinib is indicated for the treatment of patients with metastatic epidermal growth factor receptor (EGFR) T790M mutation-positive non-small cell lung cancer (NSCLC), as detected by an FDA- approved test, who have progressed on or after EGFR-TKI therapy.
Common name
Osimertinib
IUPAC name
N-(2-{[2-(dimethylamino)ethyl](methyl)amino}-4-methoxy-5-{[4-(1-methyl-1H-indol-3-yl)pyrimidin-2-yl]amino}phenyl)prop-2-enamide
SMILES
COC1=C(NC2=NC=CC(=N2)C2=CN(C)C3=C2C=CC=C3)C=C(NC(=O)C=C)C(=C1)N(C)CCN(C)C
INCHI
InChI=1S/C28H33N7O2/c1-7-27(36)30-22-16-23(26(37-6)17-25(22)34(4)15-14-33(2)3)32-28-29-13-12-21(31-28)20-18-35(5)24-11-9-8-10-19(20)24/h7-13,16-18H,1,14-15H2,2-6H3,(H,30,36)(H,29,31,32)
FORMULA
C28H33N7O2

Common name
Osimertinib
IUPAC name
N-(2-{[2-(dimethylamino)ethyl](methyl)amino}-4-methoxy-5-{[4-(1-methyl-1H-indol-3-yl)pyrimidin-2-yl]amino}phenyl)prop-2-enamide
Molecular weight
499.607
clogP
2.950
clogS
-6.865
HBond Acceptor
6
HBond Donor
2
Total Polar Surface Area
87.55
Number of Rings
4
Rotatable Bond
10
| Drug ID | Common name | Structure CAS | SMILE | Frequency |
|---|---|---|---|---|
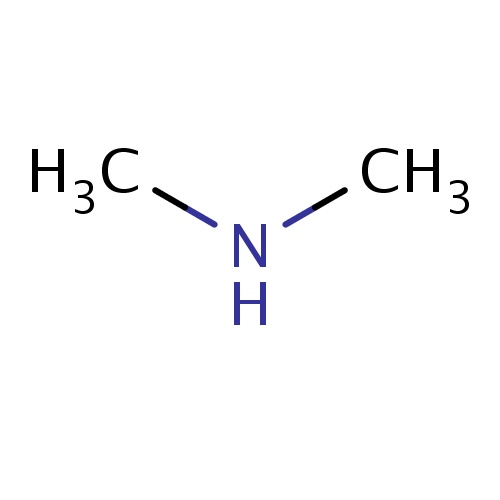
| FDBF00066 | N-methylmethanamine |
|
N(C)C | 0.0914 |
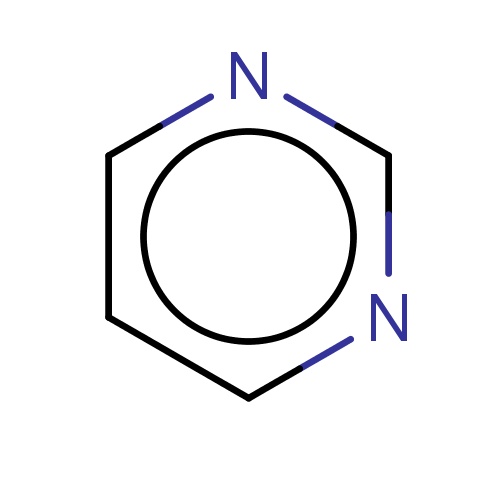
| FDBF00790 | pyrimidine |
|
n1cnccc1 | 0.0161 |
| FDBF01244 | 1-methylindole |

|
n1(c2c(cc1)cccc2)C | 0.0010 |
| FDBF04405 | 1-methyl-3-pyrimidin-4-yl-indole |

|
n1(c2c(c(c1)c3ncncc3)cccc2)C | 0.0003 |
| FDBF04407 | N-[2-(methylamino)phenyl]prop-2-enamide |

|
O=C(Nc1c(cccc1)NC)C=C | 0.0003 |

