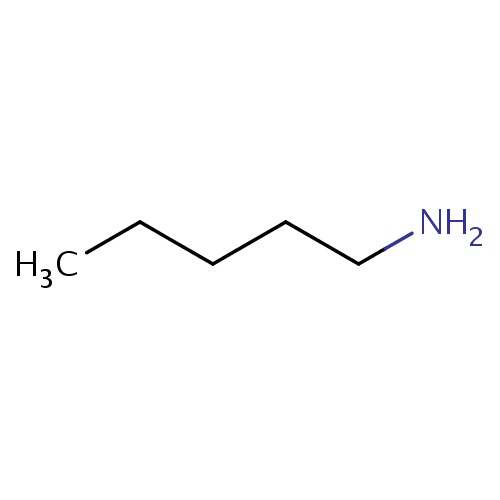
Common name
pentan-1-amine
IUPAC name
pentan-1-amine
SMILES
CCCCCN
Common name
pentan-1-amine
IUPAC name
pentan-1-amine
SMILES
CCCCCN
INCHI
InChI=1S/C5H13N/c1-2-3-4-5-6/h2-6H2,1H3
FORMULA
C5H13N

Common name
pentan-1-amine
IUPAC name
pentan-1-amine
Molecular weight
87.163
clogP
0.614
clogS
-1.424
Frequency
0.0045
HBond Acceptor
0
HBond Donor
2
Total PolarSurface Area
26.02
Number of Rings
0
Rotatable Bond
3
| Drug ID | Common name | Structure CAS | Compound class | Therapeutic area |
|---|---|---|---|---|
| FDBD00006 | Felypressin |

|
Vasoconstrictor Agents; Renal Agents; | For use as an alternative to adrenaline as a localising agent, provided that local ischaemia is not essential. |
| FDBD00195 | Ibutilide |
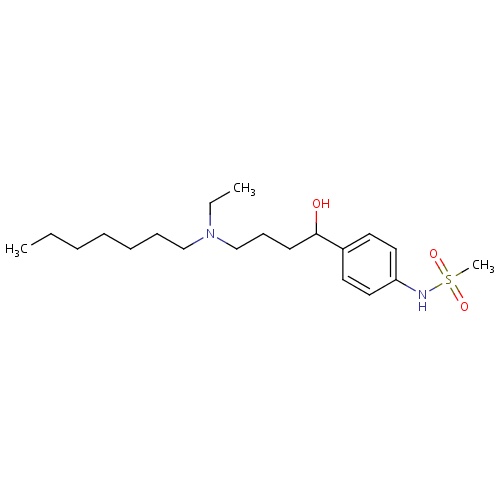
|
Anti-Arrhythmia Agents; Cardiovascular System; Antiarrhythmics, Class III; Antiarrhythmics, Class I and Iii; Cardiac Therapy; Antiarrythmics, Class I and Iii; | Indicated for the rapid conversion of atrial fibrillation or atrial flutter of recent onset to sinus rhythm. |
| FDBD00473 | Chloroquine |
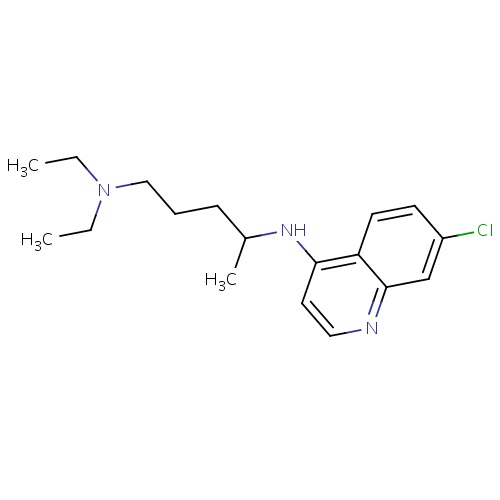
|
Antirheumatic Agents; Antimalarials; Antiprotozoal Agents; Amebicides; Antiparasitic Products, Insecticides and Repellents; Aminoquinolines; Cytochrome P-450 CYP2C8 Inhibitors; Cytochrome P-450 CYP2C8 Inducers; CYP2D6 Inducers; CYP2D6 Inducers (strong); CYP3A4 Inhibitors; | For the suppressive treatment and for acute attacks of malaria due to P. vivax, P.malariae, P. ovale, and susceptible strains of P. falciparum, Second-line agent in treatment of Rheumatoid Arthritis. |
| FDBD00586 | Lisinopril |
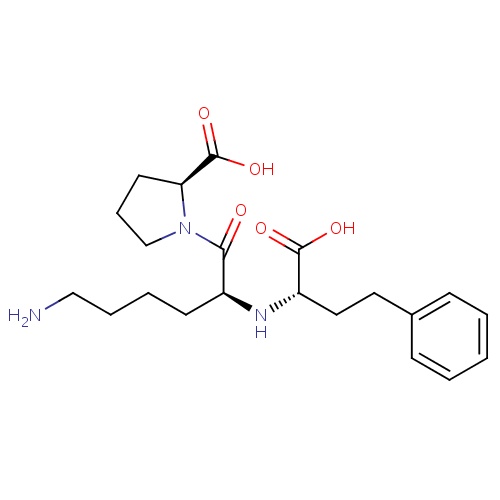
|
Angiotensin-Converting Enzyme Inhibitors; Antihypertensive Agents; Cardiotonic Agents; Lipid Modifying Agents; Cardiovascular System; Agents Acting on the Renin-Angiotensin System; ACE Inhibitors, Plain; ACE Inhibitors and Diuretics; ACE Inhibitors and Calcium Channel Blockers; | For the treatment of hypertension and symptomatic congestive heart failure. May be used in conjunction with thrombolytic agents, aspirin and/or β-blockers to improve survival in hemodynamically stable individuals following myocardial infarction. May be used to slow the progression of renal disease in hypertensive patients with diabetes mellitus and microalbuminuria or overt nephropathy. |
| FDBD00793 | Salmeterol |
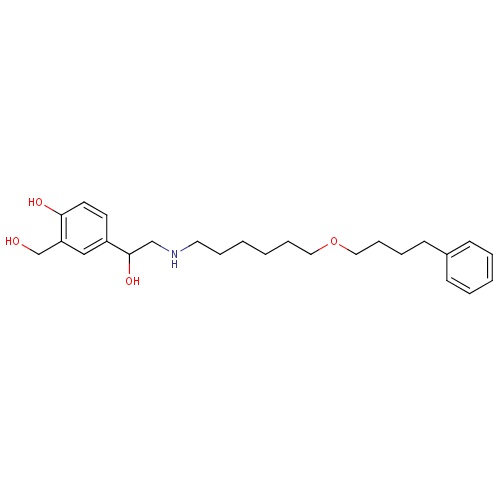
|
Sympathomimetics; Adrenergic beta-2 Receptor Agonists; Bronchodilator Agents; Respiratory System; Drugs for Obstructive Airway Diseases; Selective Beta-2-Adrenoreceptor Agonists; Adrenergics, Inhalants; Cytochrome P-450 CYP2C8 Inhibitors; Cytochrome P-450 CYP2C8 Inducers; CYP3A4 Inhibitors; Beta2 Agonists; | For the treatment of asthma and chronic obstructive pulmonary disease (COPD). |
| FDBD00937 | Primaquine |
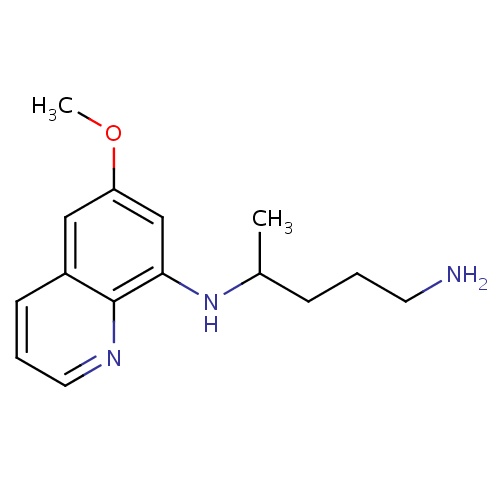
|
Antimalarials; Antiprotozoal Agents; Antiparasitic Products, Insecticides and Repellents; Aminoquinolines; Cytochrome P-450 CYP1A2 Inhibitors; Cytochrome P-450 CYP1A2 Inducers; CYP2D6 Inducers; CYP2D6 Inducers (strong); CYP3A4 Inhibitors; | For the treatment of malaria. |
| FDBD00953 | Quinacrine |
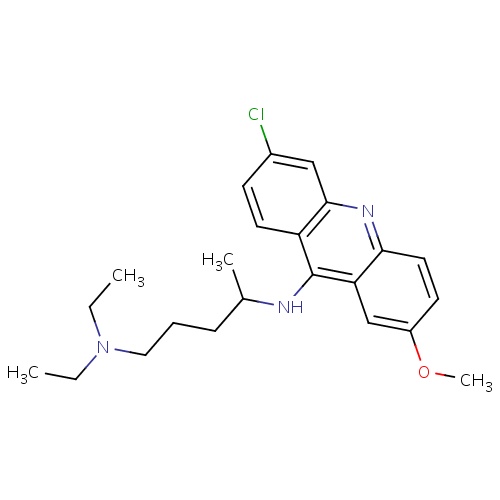
|
Antineoplastic Agents; Enzyme Inhibitors; Antimalarials; Antiprotozoal Agents; Antinematodal Agents; Anthelmintics; Anticestodal Agents; Antiparasitic Products, Insecticides and Repellents; Agents Against Protozoal Diseases; CYP3A4 Inhibitors; | For the treatment of giardiasis and cutaneous leishmaniasis and the management of malignant effusions. |
| FDBD01261 | Hydroxychloroquine |
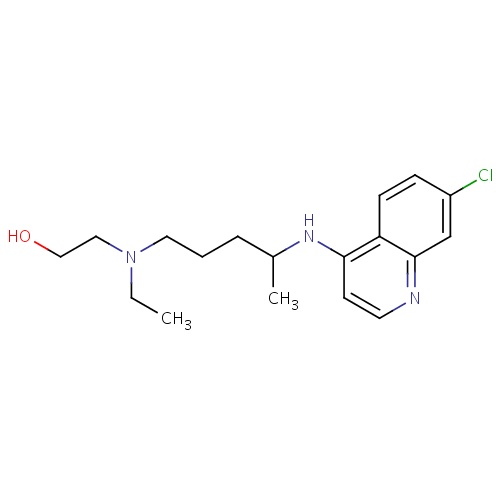
|
Antirheumatic Agents; Enzyme Inhibitors; Antimalarials; Antiprotozoal Agents; Antiparasitic Products, Insecticides and Repellents; Aminoquinolines; CYP2D6 Inducers; CYP2D6 Inducers (strong); | For the suppressive treatment and treatment of acute attacks of malaria due to . |
| FDBD01592 | Hexoprenaline |
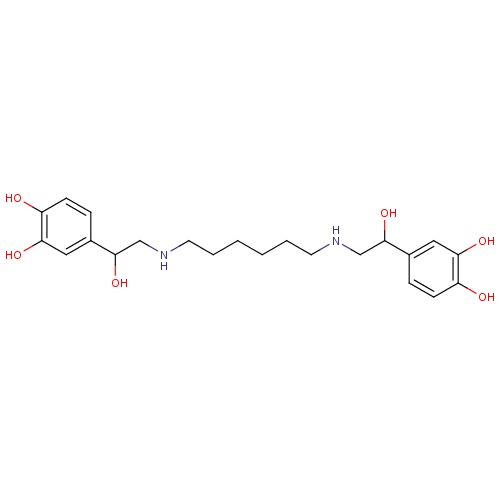
|
Adrenergic beta-2 Receptor Agonists; Bronchodilator Agents; Tocolytic Agents; Respiratory System; Drugs for Obstructive Airway Diseases; Selective Beta-2-Adrenoreceptor Agonists; Adrenergics, Inhalants; Adrenergics for Systemic Use; | |
| FDBD01665 | Vilanterol |
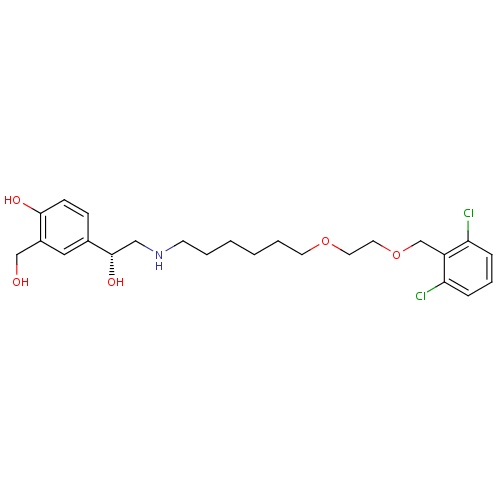
|
Immunosuppressive Agents; Respiratory System; Drugs for Obstructive Airway Diseases; Adrenergics, Inhalants; CYP3A4 Inhibitors; Beta2 Agonists; | Vilanterol is approved for use in several combination products such as with fluticasone furoate under the tradename Breo Ellipta and in combination with umeclidinium bromide as Anoro Ellipta. Approved by the FDA in 2013, use of Breo Ellipta is indicated for the long-term, once-daily maintenance treatment of airflow obstruction in patients with COPD, including chronic bronchitis and emphysema. It is also indicated for once-daily maintenance treatment of asthma in patients aged 18 or older with reversible obstructive airways disease. |
13 ,
2
| FRAGNAME | PDBID | SIMILIRITY | XSCORE | SMILE | HAC |
|---|---|---|---|---|---|
| 2v0z_ligand_4_3381.mol2 | 2v0z | 1 | -6.59 | C(C[NH3+])[C@@H](C(C)C)C | 8 |
| 2v12_ligand_4_2730.mol2 | 2v12 | 1 | -6.48 | C(C)(C)[C@H](CC[NH3+])C | 8 |
| 2v0z_ligand_3_804.mol2 | 2v0z | 1 | -6.34 | C(C[NH3+])CC(C)C | 7 |
| 2v10_ligand_4_1694.mol2 | 2v10 | 1 | -6.33 | C[C@H](C(C)C)CC[NH3+] | 8 |
100 ,
11

