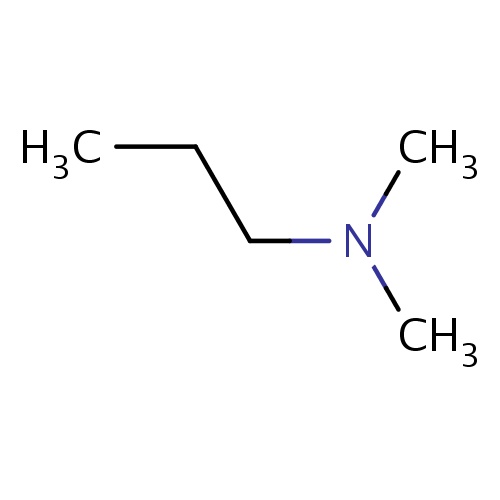
Common name
N,N-dimethylpropan-1-amine
IUPAC name
N,N-dimethylpropan-1-amine
SMILES
N(C)(C)CCC
Common name
N,N-dimethylpropan-1-amine
IUPAC name
N,N-dimethylpropan-1-amine
SMILES
N(C)(C)CCC
INCHI
InChI=1S/C5H13N/c1-4-5-6(2)3/h4-5H2,1-3H3
FORMULA
C5H13N

Common name
N,N-dimethylpropan-1-amine
IUPAC name
N,N-dimethylpropan-1-amine
Molecular weight
87.163
clogP
0.237
clogS
-1.084
Frequency
0.0113
HBond Acceptor
1
HBond Donor
0
Total PolarSurface Area
3.24
Number of Rings
0
Rotatable Bond
2
| Drug ID | Common name | Structure CAS | Compound class | Therapeutic area |
|---|---|---|---|---|
| FDBD01264 | Acepromazine |
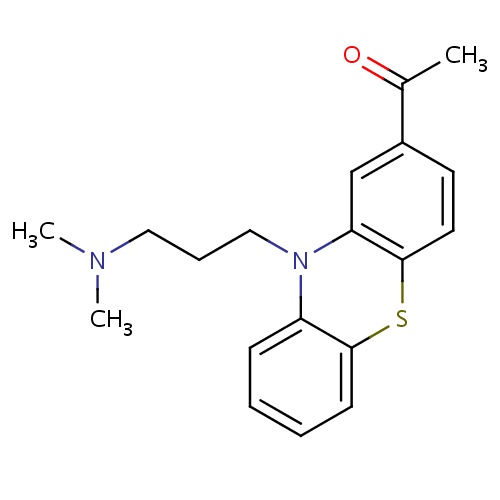
|
Hypnotics and Sedatives; Antipsychotic Agents; Adrenergic alpha-1 Receptor Antagonists; Dopamine Antagonists; Nervous System; Psycholeptics; Phenothiazines With Aliphatic Side-Chain; | Acepromazine was first used in humans in the 1950s as an antipsychotic agent. It is now rarely used in humans. Acepromazine is frequently used in animals as a sedative and antiemetic. Its principal value is in quietening and calming anxious animals. |
| FDBD01269 | Pheniramine |

|
Anti-Allergic Agents; Antipruritics; Histamine H1 Antagonists; Respiratory System; Dermatologicals; Antipruritics, Incl. Antihistamines, Anesthetics, Etc.; Antihistamines for Topical Use; Antihistamines for Systemic Use; Substituted Alkylamines; | Pheniramine is an antihistamine used to treat allergic conditions such as hay fever or urticaria. |
| FDBD01333 | Dronedarone |
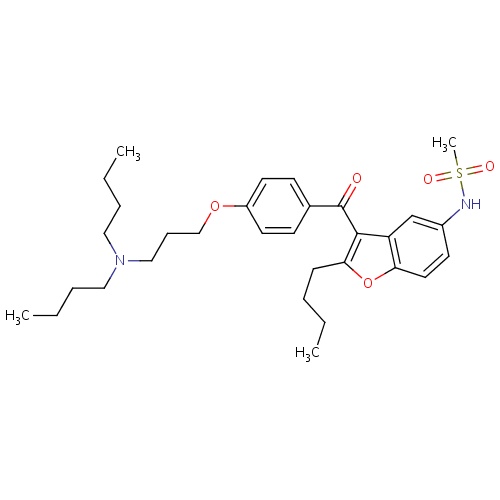
|
Anti-Arrhythmia Agents; Adrenergic alpha-1 Receptor Antagonists; Cardiovascular System; Antiarrhythmics, Class III; Antiarrhythmics, Class I and Iii; Cardiac Therapy; Antiarrythmics, Class I and Iii; CYP2D6 Inducers; CYP2D6 Inducers (strong); CYP3A4 Inhibitors; Combined Inhibitors of CYP3A4 and P-glycoprotein; | Management of paroxysmal or persistent atrial fibrillation via restoration of normal sinus rhythm. |
| FDBD01384 | Tapentadol |
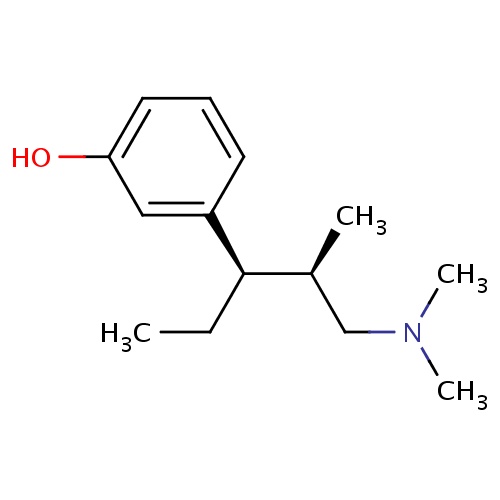
|
Analgesics; Nervous System; Opioids; Cytochrome P-450 CYP2C9 Inhibitors; Cytochrome P-450 CYP2C9 Inducers; Cytochrome P-450 CYP2C19 Inducers; CYP2D6 Inducers; CYP2D6 Inducers (strong); | The immediate-release formulation of tapentadol is indicated for the relief of moderate to severe acute pain. The long-acting formulation serves as a continuous, around-the-clock analgesic that is indicated for the relief of moderate to severe chronic pain or neuropathic pain associated with diabetic peripheral neuropathy. |
| FDBD01488 | Levopropoxyphene |
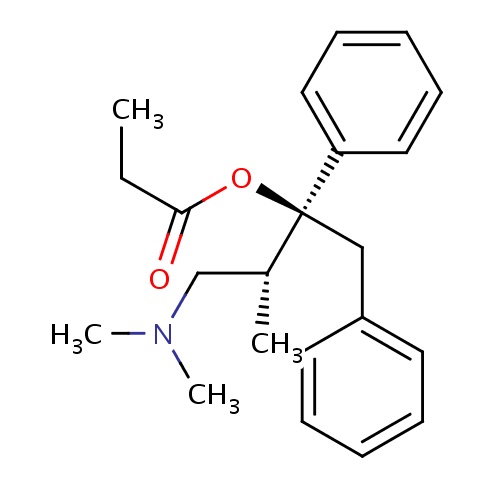
|
; | |
| FDBD01616 | Dimetacrine |

|
Nervous System; Antidepressants; Psychoanaleptics; Non-Selective Monoamine Reuptake Inhibitors; | |
| FDBD01618 | Cyamemazine |
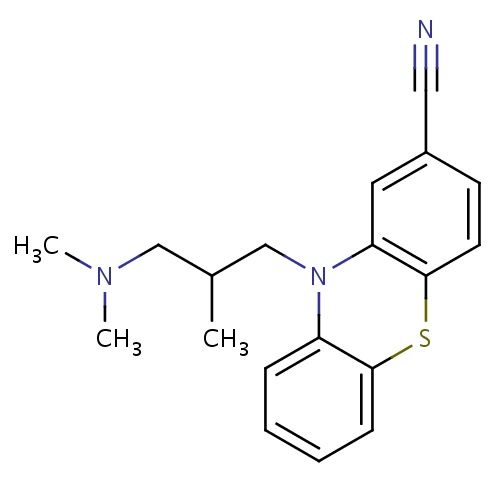
|
Antipsychotic Agents; Nervous System; Psycholeptics; Phenothiazines With Aliphatic Side-Chain; | |
| FDBD01627 | Butriptyline |
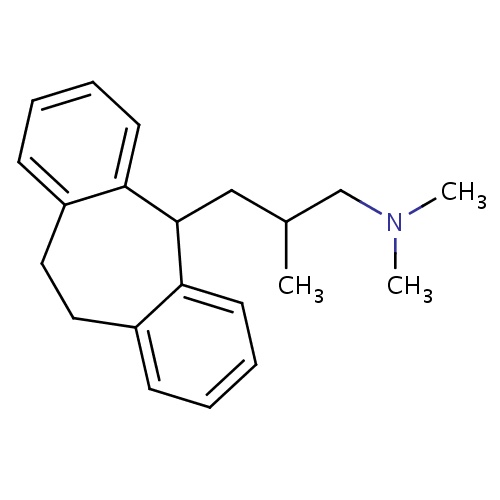
|
Nervous System; Antidepressants; Psychoanaleptics; Non-Selective Monoamine Reuptake Inhibitors; | |
| FDBD01666 | Ivabradine |

|
Cardiovascular Agents; Cardiovascular System; Cardiac Therapy; CYP3A4 Inhibitors; | Ivabradine's indication by the FDA is to reduce the risk of hospitalization for worsening heart failure in patients with stable, symptomatic chronic heart failure with left ventricular ejection fraction |
| FDBD01667 | Benzydamine |

|
Anti-Inflammatory Agents; Genito Urinary System and Sex Hormones; Alimentary Tract and Metabolism; Musculo-Skeletal System; Stomatological Preparations; Antiinflammatory and Antirheumatic Products, Non-Steroids; Antiinflammatory and Antirheumatic Products; Antiinflammatory Preparations, Non-Steroids for Topical Use; Topical Products for Joint and Muscular Pain; Antiinflammatory Products for Vaginal Administration; |
33 ,
4
| FRAGNAME | PDBID | SIMILIRITY | XSCORE | SMILE | HAC |
|---|---|---|---|---|---|
| 4bgk_ligand_3_3.mol2 | 4bgk | 1 | -6.34 | [N+](C)(C)(C)CCC | 7 |
| 4fmu_ligand_2_22.mol2 | 4fmu | 1 | -6.21 | C[NH2+]CCC | 5 |
| 1pot_ligand_3_10.mol2 | 1pot | 1 | -6.20 | C(C[NH2+]C)C | 5 |
| 3fhe_ligand_4_121.mol2 | 3fhe | 1 | -5.98 | C[NH+](CCC)C | 6 |
| 4fmu_ligand_3_22.mol2 | 4fmu | 1 | -5.98 | C([NH2+]C)CC | 5 |
| 1p0y_ligand_2_5.mol2 | 1p0y | 1 | -5.96 | C([NH2+]C)CC | 5 |
| 3k26_ligand_3_85.mol2 | 3k26 | 1 | -5.95 | C([N+](C)(C)C)CC | 7 |
| 3iiw_ligand_3_781.mol2 | 3iiw | 1 | -5.94 | C([N+](C)(C)C)CC | 7 |
| 3jzg_ligand_3_550.mol2 | 3jzg | 1 | -5.94 | CCC[N+](C)(C)C | 7 |
211 ,
22

