
Common name
morpholine
IUPAC name
morpholine
SMILES
O1CCNCC1
Common name
morpholine
IUPAC name
morpholine
SMILES
O1CCNCC1
INCHI
InChI=1S/C4H9NO/c1-3-6-4-2-5-1/h5H,1-4H2
FORMULA
C4H9NO

Common name
morpholine
IUPAC name
morpholine
Molecular weight
87.120
clogP
1.241
clogS
-0.677
Frequency
0.0082
HBond Acceptor
1
HBond Donor
1
Total PolarSurface Area
21.26
Number of Rings
1
Rotatable Bond
0
| Drug ID | Common name | Structure CAS | Compound class | Therapeutic area |
|---|---|---|---|---|
| FDBD01017 | Moclobemide |
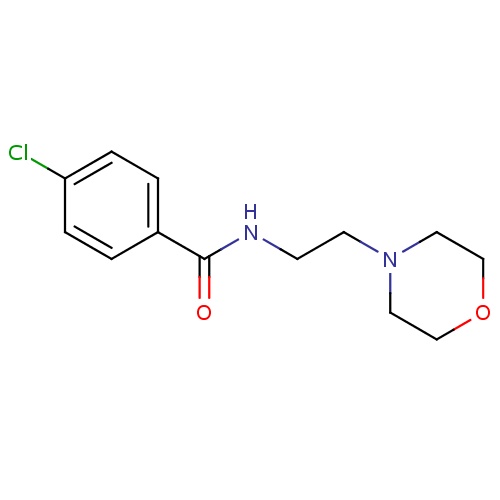
|
Antidepressive Agents; Monoamine Oxidase Inhibitors; Nervous System; Antidepressants; Psychoanaleptics; Monoamine Oxidase a Inhibitors; Cytochrome P-450 CYP1A2 Inhibitors; Cytochrome P-450 CYP2C9 Inhibitors; Cytochrome P-450 CYP1A2 Inducers; Cytochrome P-450 CYP2C9 Inducers; Cytochrome P-450 CYP2C19 Inducers; CYP2D6 Inducers; CYP2D6 Inducers (strong); | For the treatment of depression. |
| FDBD01267 | Molindone |

|
Antipsychotic Agents; Nervous System; Psycholeptics; Indole Derivatives; | Molindone is used for the management of the manifestations of psychotic disorders. |
| FDBD01457 | Fosaprepitant |

|
Antiemetics; Neurokinin-1 Receptor Antagonists; | For the prevention of nausea and vomiting associated with highly emetogenic cancer chemotherapy. |
| FDBD01557 | Carfilzomib |

|
Antineoplastic Agents; Antineoplastic and Immunomodulating Agents; | Carfilzomib is indicated for the treatment of patients with multiple myeloma who have received at least two prior therapies including bortezomib and an immunomodulatory agent and have demonstrated disease progression on or within 60 days of completion of the last therapy. Approval is based on response rate. |
| FDBD01652 | Cobicistat |
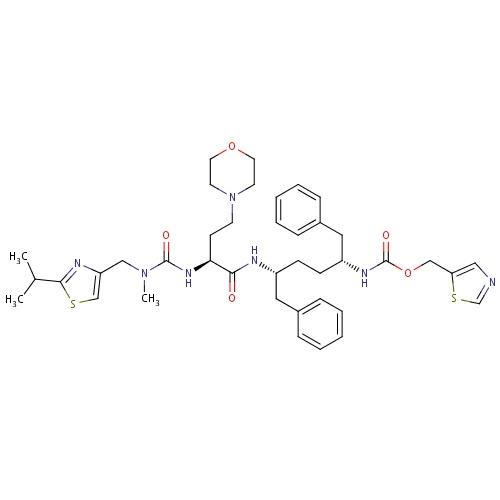
|
Anti-HIV Agents; Antiinfectives for Systemic Use; Direct Acting Antivirals; Antivirals for Systemic Use; Cytochrome P-450 CYP3A Inhibitors; CYP2D6 Inducers; CYP2D6 Inducers (strong); | Cobicistat is a CYP3A inhibitor indicated to increase systemic exposure of atazanavir or darunavir (once daily dosing regimen) in combination with other antiretroviral agents in the treatment of HIV-1 infection. It is not interchangeable with ritonavir to increase systemic exposure of darunavir 600 mg twice daily, fosamprenavir, saquinavir, or tipranavir due to lack of exposure data. The use of cobicistat is not recommended with darunavir 600 mg twice daily, fosamprenavir, saquinavir or tipranavir. Complex or unknown mechanisms of drug interactions preclude extrapolation of ritonavir drug interactions to certain cobicistat interactions. Cobicistat and ritonavir when administered with either atazanavir or darunavir may result in different drug interactions when used with concomitant medications. |
| FDBD01672 | Pinaverium |
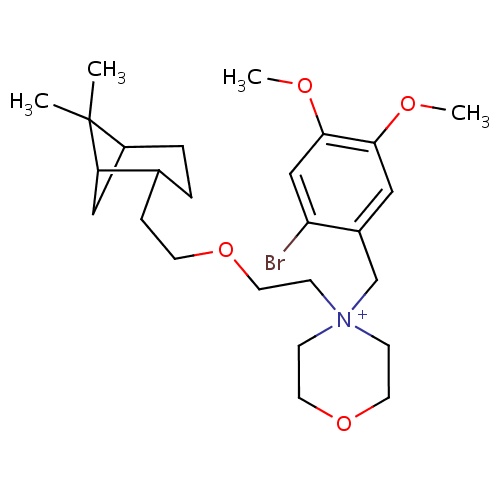
|
Alimentary Tract and Metabolism; Drugs for Functional Gastrointestinal Disorders; | |
| FDBD01732 | Viloxazine |

|
Antidepressive Agents, Second-Generation; Adrenergic Uptake Inhibitors; Nervous System; Antidepressants; Psychoanaleptics; | For the treatment of clinical depression. |
| FDBD01739 | Pholcodine |

|
Cough and Cold Preparations; Respiratory System; Opium Alkaloids and Derivatives; | For use as a cough suppressant. |
| FDBD01780 | Molsidomine |
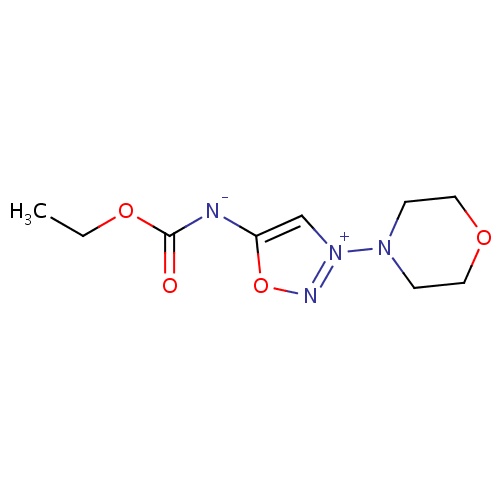
|
Vasodilator Agents; Nitric Oxide Donors; Cardiovascular System; Cardiac Therapy; Vasodilators Used in Cardiac Diseases; | |
| FDBD01783 | Morniflumate |
|
Musculo-Skeletal System; Antiinflammatory and Antirheumatic Products, Non-Steroids; Antiinflammatory and Antirheumatic Products; |
24 ,
3
| FRAGNAME | PDBID | SIMILIRITY | XSCORE | SMILE | HAC |
|---|---|---|---|---|---|
| 4uwh_ligand_frag_2.mol2 | 4uwh | 1 | -5.88 | C1COCC[NH2+]1 | 6 |
| 4uwf_ligand_frag_2.mol2 | 4uwf | 1 | -5.86 | C1COCC[NH2+]1 | 6 |
| 2wxl_ligand_frag_0.mol2 | 2wxl | 1 | -5.85 | C1COCC[NH2+]1 | 6 |
| 4bfr_ligand_frag_1.mol2 | 4bfr | 1 | -5.84 | [NH2+]1CCOCC1 | 6 |
| 4urk_ligand_frag_1.mol2 | 4urk | 1 | -5.81 | [NH2+]1CCOCC1 | 6 |
| 4f6u_ligand_frag_6.mol2 | 4f6u | 1 | -5.78 | [NH2+]1CCOCC1 | 6 |
| 4l23_ligand_frag_1.mol2 | 4l23 | 1 | -5.78 | [NH2+]1CCOCC1 | 6 |
| 3apc_ligand_frag_0.mol2 | 3apc | 1 | -5.77 | C1COCC[NH2+]1 | 6 |
165 ,
17

