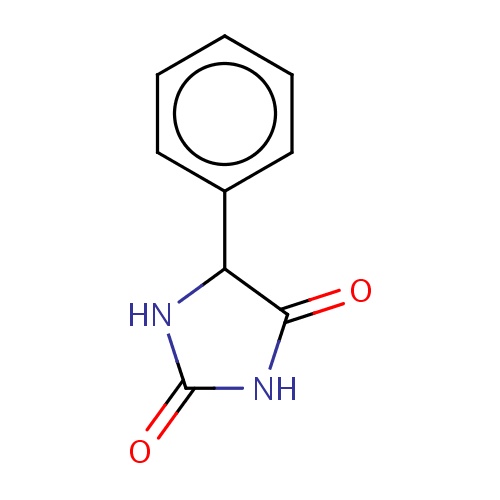
Common name
(5S)-5-phenylimidazolidine-2,4-dione
IUPAC name
(5S)-5-phenylimidazolidine-2,4-dione
SMILES
c1(ccccc1)C2C(=O)NC(=O)N2
Common name
(5S)-5-phenylimidazolidine-2,4-dione
IUPAC name
(5S)-5-phenylimidazolidine-2,4-dione
SMILES
c1(ccccc1)C2C(=O)NC(=O)N2
INCHI
InChI=1S/C9H8N2O2/c12-8-7(10-9(13)11-8)6-4-2-1-3-5-6/h1-5,7H,(H2,10,11,12,13)/t7-/m0/s1
FORMULA
C9H8N2O2

Common name
(5S)-5-phenylimidazolidine-2,4-dione
IUPAC name
(5S)-5-phenylimidazolidine-2,4-dione
Molecular weight
176.172
clogP
0.929
clogS
-2.118
Frequency
0.0007
HBond Acceptor
2
HBond Donor
2
Total PolarSurface Area
58.2
Number of Rings
2
Rotatable Bond
1
| Drug ID | Common name | Structure CAS | Compound class | Therapeutic area |
|---|---|---|---|---|
| FDBD00140 | Phenytoin |
|
Anticonvulsants; Voltage-Gated Sodium Channel Blockers; Nervous System; Antiepileptics; Hydantoin Derivatives; Cytochrome P-450 CYP2C9 Inhibitors; Cytochrome P-450 CYP2C8 Inhibitors; Cytochrome P-450 CYP2C9 Inducers; Cytochrome P-450 CYP2C19 Inducers; Cytochrome P-450 CYP2C8 Inducers; Cytochrome P-450 CYP2B6 Inducers; Cytochrome P-450 CYP2B6 Inhibitors; CYP2B6 Inhibitors (strong); CYP3A4 Inhibitors; | For the control of generalized tonic-clonic (grand mal) and complex partial (psychomotor, temporal lobe) seizures and prevention and treatment of seizures occurring during or following neurosurgery. |
| FDBD01128 | Fosphenytoin |

|
Anticonvulsants; Nervous System; Antiepileptics; Hydantoin Derivatives; Cytochrome P-450 CYP2C9 Inhibitors; Cytochrome P-450 CYP2C8 Inhibitors; Cytochrome P-450 CYP2C9 Inducers; Cytochrome P-450 CYP2C19 Inducers; Cytochrome P-450 CYP2C8 Inducers; Cytochrome P-450 CYP2B6 Inducers; Cytochrome P-450 CYP2B6 Inhibitors; CYP2B6 Inhibitors (strong); CYP3A4 Inhibitors; | For the control of generalized convulsive status epilepticus and prevention and treatment of seizures occurring during neurosurgery. It can also be substituted, short-term, for oral phenytoin. |
2 ,
1
| FRAGNAME | PDBID | SIMILIRITY | XSCORE | SMILE | HAC |
|---|---|---|---|---|---|
| 3eq9_ligand_1_5.mol2 | 3eq9 | 1 | -6.41 | c1(ccccc1)[C@@H]1NC(=O)NC1=O | 13 |
| 3eq9_ligand_1_4.mol2 | 3eq9 | 1 | -6.02 | N1C(=O)N[C@@H](C1=O)c1ccccc1 | 13 |
| 3eq9_ligand_2_16.mol2 | 3eq9 | 0.957746 | -6.52 | CN1C(=O)N[C@@H](c2ccccc2)C1=O | 14 |
| 3eq9_ligand_2_15.mol2 | 3eq9 | 0.957746 | -6.13 | CN1C(=O)N[C@@H](C1=O)c1ccccc1 | 14 |
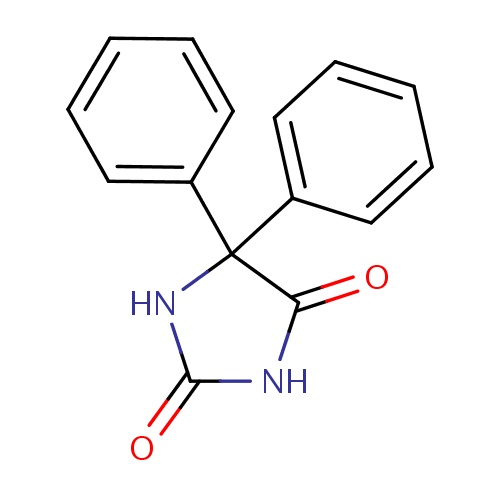
| 3eq9_ligand_2_18.mol2 | 3eq9 | 0.944444 | -7.08 | c1(ccccc1)C1(NC(=O)NC1=O)c1ccccc1 | 19 |
| 3eq9_ligand_3_31.mol2 | 3eq9 | 0.906667 | -7.19 | CN1C(=O)NC(c2ccccc2)(C1=O)c1ccccc1 | 20 |
108 ,
11

