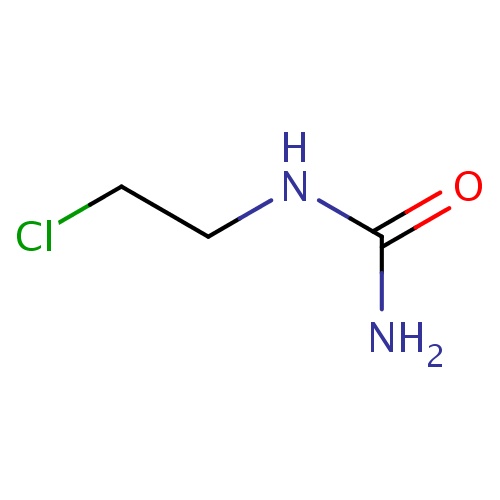
Common name
2-chloroethylurea
IUPAC name
2-chloroethylurea
SMILES
ClCCNC(=O)N
Common name
2-chloroethylurea
IUPAC name
2-chloroethylurea
SMILES
ClCCNC(=O)N
INCHI
InChI=1S/C3H7ClN2O/c4-1-2-6-3(5)7/h1-2H2,(H3,5,6,7)
FORMULA
C3H7ClN2O

Common name
2-chloroethylurea
IUPAC name
2-chloroethylurea
Molecular weight
122.553
clogP
-0.334
clogS
-0.901
Frequency
0.0007
HBond Acceptor
1
HBond Donor
3
Total PolarSurface Area
55.12
Number of Rings
0
Rotatable Bond
2
| Drug ID | Common name | Structure CAS | Compound class | Therapeutic area |
|---|---|---|---|---|
| FDBD00150 | Carmustine |
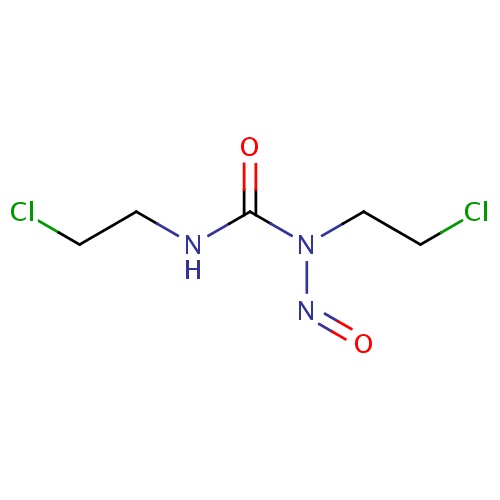
|
Antineoplastic Agents; Immunosuppressive Agents; Antineoplastic Agents, Alkylating; Alkylating Agents; Antineoplastic and Immunomodulating Agents; Nitrosoureas; Cytochrome P-450 CYP1A2 Inhibitors; Cytochrome P-450 CYP1A2 Inducers; | For the treatment of brain tumors, multiple myeloma, Hodgkin's disease and Non-Hodgkin's lymphomas. |
| FDBD01052 | Lomustine |
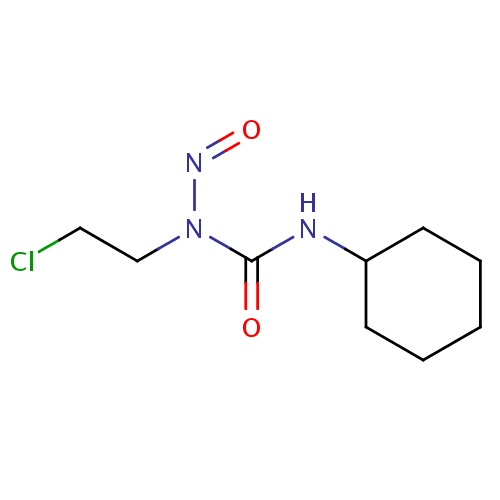
|
Antineoplastic Agents; Immunosuppressive Agents; Antineoplastic Agents, Alkylating; Alkylating Agents; Antineoplastic and Immunomodulating Agents; Nitrosoureas; CYP2D6 Inducers; CYP2D6 Inducers (strong); CYP3A4 Inhibitors; | For the treatment of primary and metastatic brain tumors as a component of combination chemotherapy in addition to appropriate surgical and/or radiotherapeutic procedures. Also used in combination with other agents as secondary therapy for the treatment of refractory or relapsed Hodgkin's disease. |
2 ,
1
| FRAGNAME | PDBID | SIMILIRITY | XSCORE | SMILE | HAC |
|---|---|---|---|---|---|
| 2gde_ligand_2_45.mol2 | 2gde | 0.789474 | -5.15 | N(C=O)CCCl | 6 |
| 2gde_ligand_3_186.mol2 | 2gde | 0.681818 | -5.30 | N(C(=O)C)CCCl | 7 |
| 2yix_ligand_1_0.mol2 | 2yix | 0.631579 | -6.13 | CCNC(=O)N | 6 |
| 1zd3_ligand_2_3.mol2 | 1zd3 | 0.631579 | -6.12 | C(C)NC(=O)N | 6 |
| 4lpb_ligand_1_0.mol2 | 4lpb | 0.631579 | -6.07 | C(C)NC(=O)N | 6 |
| 4lp0_ligand_1_0.mol2 | 4lp0 | 0.631579 | -6.04 | C(C)NC(=O)N | 6 |
115 ,
12

