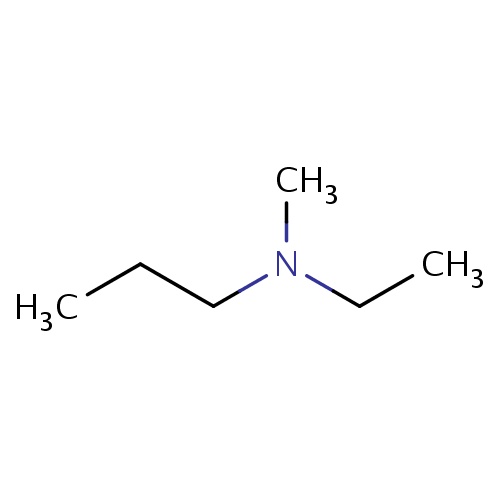
Common name
N-ethyl-N-methyl-propan-1-amine
IUPAC name
N-ethyl-N-methyl-propan-1-amine
SMILES
C(C)CN(C)CC
Common name
N-ethyl-N-methyl-propan-1-amine
IUPAC name
N-ethyl-N-methyl-propan-1-amine
SMILES
C(C)CN(C)CC
INCHI
InChI=1S/C6H15N/c1-4-6-7(3)5-2/h4-6H2,1-3H3
FORMULA
C6H15N

Common name
N-ethyl-N-methyl-propan-1-amine
IUPAC name
N-ethyl-N-methyl-propan-1-amine
Molecular weight
101.190
clogP
0.662
clogS
-1.515
Frequency
0.0031
HBond Acceptor
1
HBond Donor
0
Total PolarSurface Area
3.24
Number of Rings
0
Rotatable Bond
3
| Drug ID | Common name | Structure CAS | Compound class | Therapeutic area |
|---|---|---|---|---|
| FDBD00156 | Ropinirole |
|
Antiparkinson Agents; Dopamine Agonists; Antidyskinetics; Nervous System; Anti-Parkinson Drugs; Dopaminergic Agents; Cytochrome P-450 CYP1A2 Inhibitors; Cytochrome P-450 CYP1A2 Inducers; CYP2D6 Inducers; CYP2D6 Inducers (strong); CYP3A4 Inhibitors; Alpha2 Agonists; | For the treatment of the signs and symptoms of idiopathic Parkinson's disease. Also used for the treatment of restless legs syndrome. |
| FDBD00195 | Ibutilide |
|
Anti-Arrhythmia Agents; Cardiovascular System; Antiarrhythmics, Class III; Antiarrhythmics, Class I and Iii; Cardiac Therapy; Antiarrythmics, Class I and Iii; | Indicated for the rapid conversion of atrial fibrillation or atrial flutter of recent onset to sinus rhythm. |
| FDBD00526 | Verapamil |
|
Anti-Arrhythmia Agents; Vasodilator Agents; Calcium Channel Blockers; Cardiovascular System; Agents Acting on the Renin-Angiotensin System; ACE Inhibitors and Calcium Channel Blockers; Selective Calcium Channel Blockers With Direct Cardiac Effects; Phenylalkylamine Derivatives; Cytochrome P-450 CYP1A2 Inhibitors; Cytochrome P-450 CYP2C9 Inhibitors; Cytochrome P-450 CYP1A2 Inducers; Cytochrome P-450 CYP2C8 Inhibitors; Cytochrome P-450 CYP2C9 Inducers; Cytochrome P-450 CYP2C19 Inducers; Cytochrome P-450 CYP2C8 Inducers; Cytochrome P-450 CYP2B6 Inducers; Cytochrome P-450 CYP2B6 Inhibitors; CYP2B6 Inhibitors (strong); CYP2D6 Inducers; CYP2D6 Inducers (strong); CYP3A4 Inhibitors; BSEP/ABCB11 Inhibitors; Combined Inhibitors of CYP3A4 and P-glycoprotein; | For the treatment of hypertension, angina, and cluster headache prophylaxis. |
| FDBD00574 | Ibandronate |

|
Bone Density Conservation Agents; Antihypocalcemic Agents; Antiresorptives; Bisphosphonates; Musculo-Skeletal System; Drugs Affecting Bone Structure and Mineralization; Drugs for Treatment of Bone Diseases; | For the treatment and prevention of osteoporosis in postmenopausal women. |
| FDBD01261 | Hydroxychloroquine |
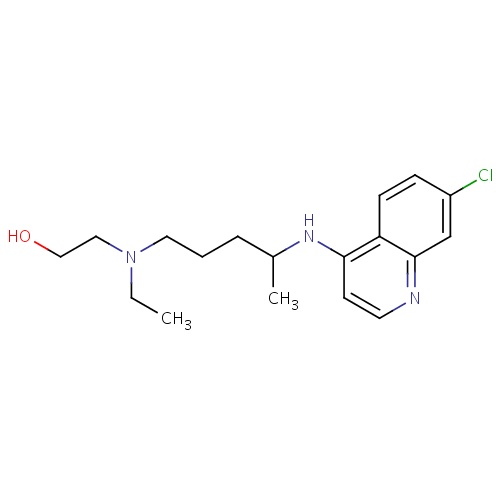
|
Antirheumatic Agents; Enzyme Inhibitors; Antimalarials; Antiprotozoal Agents; Antiparasitic Products, Insecticides and Repellents; Aminoquinolines; CYP2D6 Inducers; CYP2D6 Inducers (strong); | For the suppressive treatment and treatment of acute attacks of malaria due to . |
| FDBD01266 | Alverine |
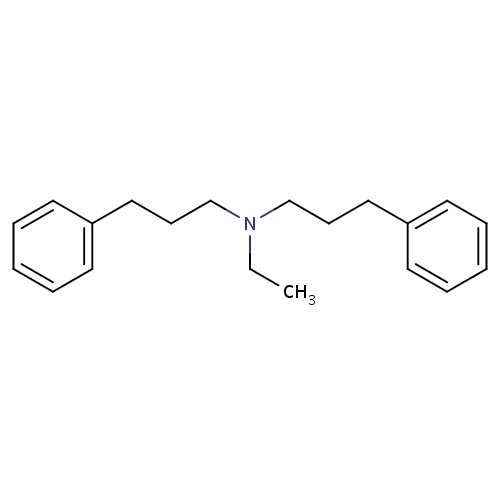
|
Parasympatholytics; Antispasmodics; Alimentary Tract and Metabolism; Drugs for Functional Gastrointestinal Disorders; | Used to relieve cramps or spasms of the stomach and intestines. It is also useful in treating irritable bowel syndrome (IBS) and similar conditions. It can also be used to help relieve period pain. Alverine citrate is also under investigation to increase the cytotoxic effects of the proteasome inhibitor MG132 on breast cancer cells. |
| FDBD01333 | Dronedarone |
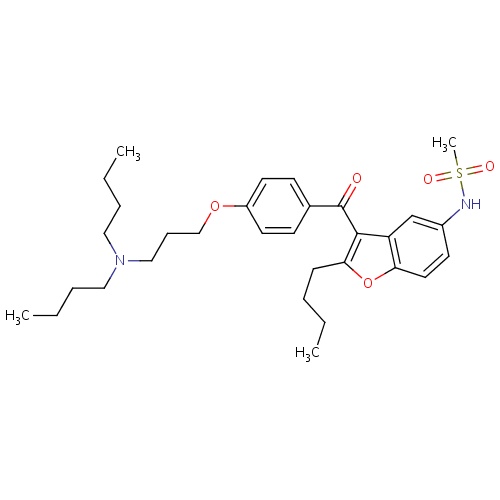
|
Anti-Arrhythmia Agents; Adrenergic alpha-1 Receptor Antagonists; Cardiovascular System; Antiarrhythmics, Class III; Antiarrhythmics, Class I and Iii; Cardiac Therapy; Antiarrythmics, Class I and Iii; CYP2D6 Inducers; CYP2D6 Inducers (strong); CYP3A4 Inhibitors; Combined Inhibitors of CYP3A4 and P-glycoprotein; | Management of paroxysmal or persistent atrial fibrillation via restoration of normal sinus rhythm. |
| FDBD01609 | Etidocaine |

|
Anesthetics, Local; Anesthetics; Nervous System; Amides; | |
| FDBD03266 | spiroxamine |
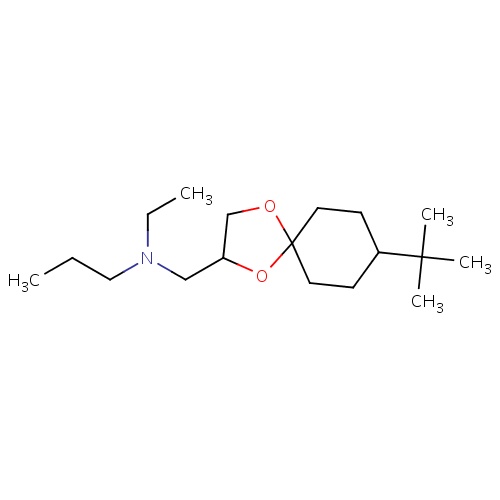
|
Fungicide | Fungicide |
9 ,
1
| FRAGNAME | PDBID | SIMILIRITY | XSCORE | SMILE | HAC |
|---|---|---|---|---|---|
| 4fmu_ligand_4_55.mol2 | 4fmu | 1 | -6.92 | C([NH2+]CCC)(C)C | 7 |
| 1pot_ligand_4_10.mol2 | 1pot | 1 | -6.61 | C(C[NH2+]CC)C | 6 |
| 4fmu_ligand_3_40.mol2 | 4fmu | 1 | -6.54 | C([NH2+]CCC)C | 6 |
| 4fmu_ligand_3_46.mol2 | 4fmu | 1 | -6.50 | C([NH2+]CCC)C | 6 |
| 3acx_ligand_3_37.mol2 | 3acx | 1 | -6.26 | C(C)(C)[NH2+]CCC | 7 |
| 4eki_ligand_5_231.mol2 | 4eki | 1 | -6.18 | C(C)C[N@H+](C)C(C)C | 8 |
| 4eki_ligand_4_175.mol2 | 4eki | 1 | -6.17 | C(C)C[NH2+]C(C)C | 7 |
| 2f94_ligand_5_50.mol2 | 2f94 | 1 | -6.12 | C(C)[N@H+](CCC)C | 7 |
| 4zun_ligand_4_20.mol2 | 4zun | 1 | -6.09 | [NH2+](CC)CCC | 6 |
274 ,
28

