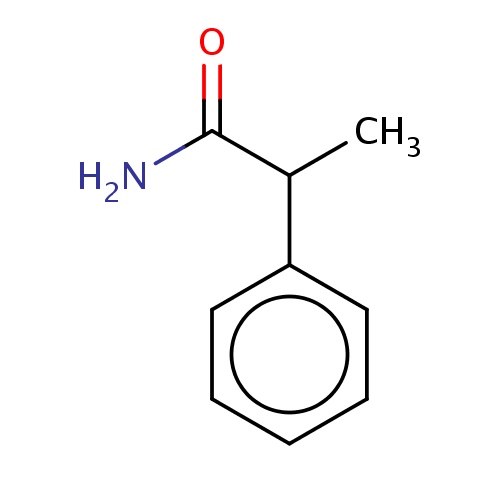
Common name
(2R)-2-phenylpropanamide
IUPAC name
(2R)-2-phenylpropanamide
SMILES
O=C(N)C(c1ccccc1)C
Common name
(2R)-2-phenylpropanamide
IUPAC name
(2R)-2-phenylpropanamide
SMILES
O=C(N)C(c1ccccc1)C
INCHI
InChI=1S/C9H11NO/c1-7(9(10)11)8-5-3-2-4-6-8/h2-7H,1H3,(H2,10,11)/t7-/m1/s1
FORMULA
C9H11NO

Common name
(2R)-2-phenylpropanamide
IUPAC name
(2R)-2-phenylpropanamide
Molecular weight
149.190
clogP
1.469
clogS
-1.646
Frequency
0.0010
HBond Acceptor
1
HBond Donor
2
Total PolarSurface Area
43.09
Number of Rings
1
Rotatable Bond
2
| Drug ID | Common name | Structure CAS | Compound class | Therapeutic area |
|---|---|---|---|---|
| FDBD00167 | Disopyramide |
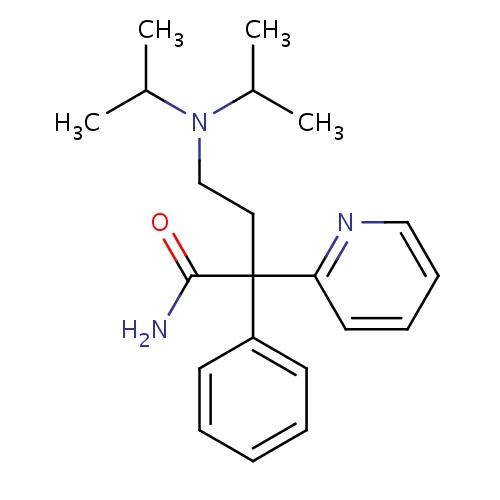
|
Anti-Arrhythmia Agents; Voltage-Gated Sodium Channel Blockers; Cardiovascular System; Antiarrhythmics, Class I and Iii; Cardiac Therapy; Antiarrythmics, Class I and Iii; Antiarrhythmics, Class Ia; CYP3A4 Inhibitors; | For the treatment of documented ventricular arrhythmias, such as sustained ventricular tachycardia, ventricular pre-excitation and cardiac dysrhythmias. It is a Class Ia antiarrhythmic drug. |
| FDBD01274 | Isopropamide |
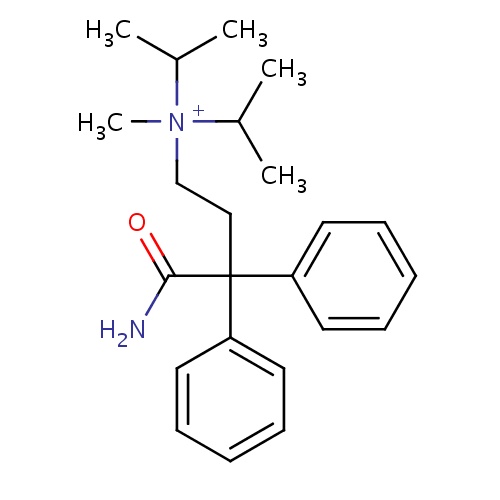
|
Alimentary Tract and Metabolism; Drugs for Functional Gastrointestinal Disorders; Synthetic Anticholinergics, Quaternary Ammonium Compounds; | For the treatment of a wide range of gastrointestinal disorders, including such conditions as peptic ulcer, gastritis, hyperchlorhydria, functional diarrhea, irritable or spastic colon, pyloroduodenal irritability, pylorospasm, acute nonspecific gastroenteritis, biliary dyskinesia and chronic cholelithiasis, duodenitis, gastrointestinal spasm; it may also be used to treat genitourinary spasm. |
| FDBD01768 | Imidafenacin |
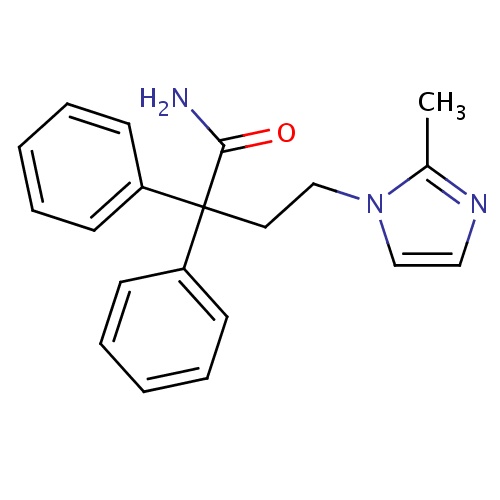
|
; |
3 ,
1
| FRAGNAME | PDBID | SIMILIRITY | XSCORE | SMILE | HAC |
|---|---|---|---|---|---|
| 3ahn_ligand_2_0.mol2 | 3ahn | 1 | -6.99 | c1(ccccc1)CC(=O)N | 10 |
| 1hte_ligand_2_20.mol2 | 1hte | 1 | -6.71 | c1c(cccc1)CC(=O)N | 10 |
| 1htf_ligand_2_56.mol2 | 1htf | 1 | -6.62 | c1ccccc1CC(=O)N | 10 |
| 1htg_ligand_2_54.mol2 | 1htg | 1 | -6.62 | C(=O)(N)Cc1ccccc1 | 10 |
| 4kqq_ligand_2_14.mol2 | 4kqq | 1 | -6.61 | c1(ccccc1)CC(=O)N | 10 |
| 4kqr_ligand_2_14.mol2 | 4kqr | 1 | -6.56 | O=C(N)Cc1ccccc1 | 10 |
| 4kqo_ligand_2_14.mol2 | 4kqo | 1 | -6.54 | c1(ccccc1)CC(=O)N | 10 |
| 4nie_ligand_2_49.mol2 | 4nie | 1 | -6.53 | C(c1ccccc1)C(=O)N | 10 |
188 ,
19

