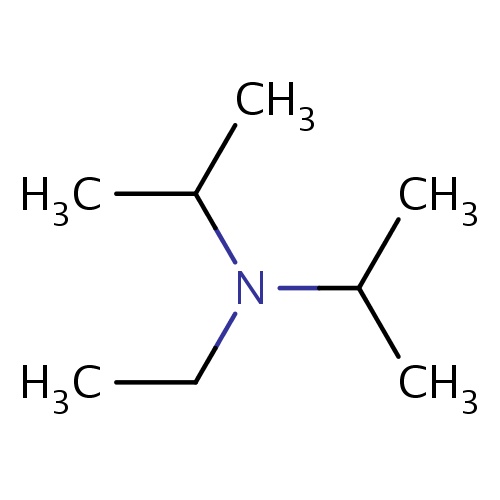
Common name
N-ethyl-N-isopropyl-propan-2-amine
IUPAC name
N-ethyl-N-isopropyl-propan-2-amine
SMILES
N(CC)(C(C)C)C(C)C
Common name
N-ethyl-N-isopropyl-propan-2-amine
IUPAC name
N-ethyl-N-isopropyl-propan-2-amine
SMILES
N(CC)(C(C)C)C(C)C
INCHI
InChI=1S/C8H19N/c1-6-9(7(2)3)8(4)5/h7-8H,6H2,1-5H3
FORMULA
C8H19N

Common name
N-ethyl-N-isopropyl-propan-2-amine
IUPAC name
N-ethyl-N-isopropyl-propan-2-amine
Molecular weight
129.243
clogP
1.185
clogS
-1.616
Frequency
0.0010
HBond Acceptor
1
HBond Donor
0
Total PolarSurface Area
3.24
Number of Rings
0
Rotatable Bond
3
| Drug ID | Common name | Structure CAS | Compound class | Therapeutic area |
|---|---|---|---|---|
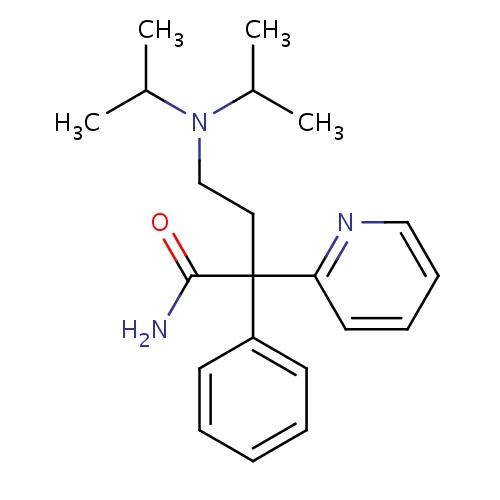
| FDBD00167 | Disopyramide |
|
Anti-Arrhythmia Agents; Voltage-Gated Sodium Channel Blockers; Cardiovascular System; Antiarrhythmics, Class I and Iii; Cardiac Therapy; Antiarrythmics, Class I and Iii; Antiarrhythmics, Class Ia; CYP3A4 Inhibitors; | For the treatment of documented ventricular arrhythmias, such as sustained ventricular tachycardia, ventricular pre-excitation and cardiac dysrhythmias. It is a Class Ia antiarrhythmic drug. |
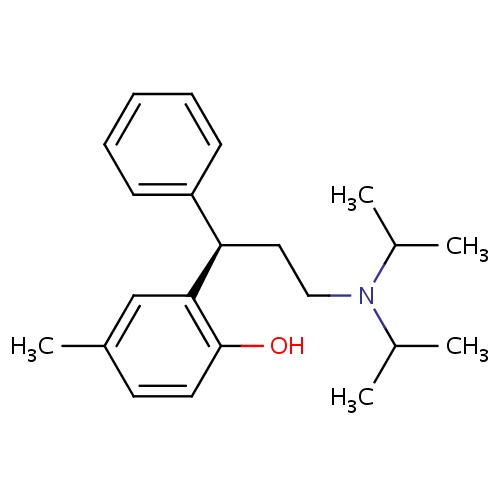
| FDBD00889 | Tolterodine |
|
Muscarinic Antagonists; Anti-Incontinence Agents; Antispasmodics; Muscle Relaxants, Genitourinary; Genito Urinary System and Sex Hormones; Drugs for Urinary Frequency and Incontinence; Cytochrome P-450 CYP2C9 Inhibitors; Urological Agents; Cytochrome P-450 CYP2C9 Inducers; Cytochrome P-450 CYP2C19 Inducers; CYP2D6 Inducers; CYP2D6 Inducers (strong); CYP3A4 Inhibitors; | For the treatment of overactive bladder (with symptoms of urinary frequency, urgency, or urge incontinence). |
| FDBD01442 | Fesoterodine |

|
Muscarinic Antagonists; Anti-Incontinence Agents; Antispasmodics; Muscle Relaxants, Genitourinary; Genito Urinary System and Sex Hormones; Drugs for Urinary Frequency and Incontinence; Urological Agents; CYP2D6 Inducers; CYP2D6 Inducers (strong); CYP3A4 Inhibitors; | For the treatment of overactive bladder (with symptoms of urinary frequency, urgency, or urge incontinence). |
3 ,
1
| FRAGNAME | PDBID | SIMILIRITY | XSCORE | SMILE | HAC |
|---|---|---|---|---|---|
| 3acx_ligand_2_15.mol2 | 3acx | 1 | -6.07 | C(C)(C)[NH2+]CC | 6 |
| 4eki_ligand_3_100.mol2 | 4eki | 1 | -6.04 | C(C)(C)[NH2+]CC | 6 |
| 4eki_ligand_4_195.mol2 | 4eki | 1 | -6.04 | CC[N@H+](C)C(C)C | 7 |
| 2c4f_ligand_2_30.mol2 | 2c4f | 1 | -5.89 | [NH2+](C(C)C)C(C)C | 7 |
| 4wvl_ligand_3_340.mol2 | 4wvl | 1 | -5.85 | C(C)(C)[NH2+]CC | 6 |
| 4wvl_ligand_4_1325.mol2 | 4wvl | 1 | -5.80 | CC[N@H+](C)C(C)C | 7 |
| 2fwz_ligand_2_0.mol2 | 2fwz | 1 | -5.47 | CC[NH2+]C(C)C | 6 |
| 3myg_ligand_3_25.mol2 | 3myg | 1 | -5.36 | C(C)[N@H+](C)C(C)C | 7 |
| 4bhf_ligand_2_1.mol2 | 4bhf | 0.857143 | -6.47 | [N+](C)(C)(C)C(C)C | 7 |
280 ,
29

