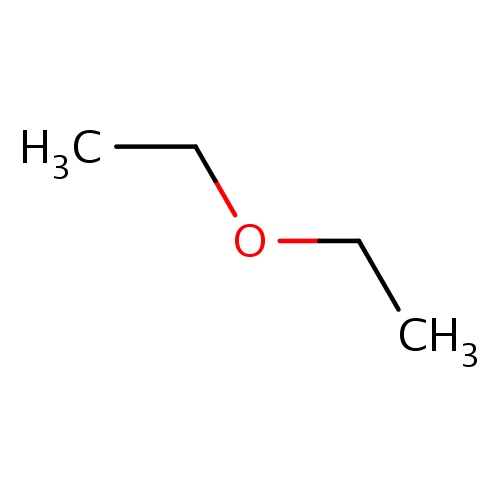
Common name
ethoxyethane
IUPAC name
ethoxyethane
SMILES
O(CC)CC
Common name
ethoxyethane
IUPAC name
ethoxyethane
SMILES
O(CC)CC
INCHI
InChI=1S/C4H10O/c1-3-5-4-2/h3-4H2,1-2H3
FORMULA
C4H10O

Common name
ethoxyethane
IUPAC name
ethoxyethane
Molecular weight
74.122
clogP
0.585
clogS
-1.070
Frequency
0.0058
HBond Acceptor
1
HBond Donor
0
Total Polar
Surface Area
9.23
Number of Rings
0
Rotatable Bond
2
| Drug ID | Common name | Structure CAS | Compound class | Therapeutic area |
|---|---|---|---|---|
| FDBD00170 | Clemastine |
|
Anti-Allergic Agents; Antipruritics; Histamine H1 Antagonists; Respiratory System; Dermatologicals; Antipruritics, Incl. Antihistamines, Anesthetics, Etc.; Antihistamines for Topical Use; Aminoalkyl Ethers; Antihistamines for Systemic Use; CYP2D6 Inducers; CYP2D6 Inducers (strong); CYP3A4 Inhibitors; | For the relief of symptoms associated with allergic rhinitis such as sneezing, rhinorrhea, pruritus and acrimation. Also for the management of mild, uncomplicated allergic skin manifestations of urticaria and angioedema. Used as self-medication for temporary relief of symptoms associated with the common cold. |
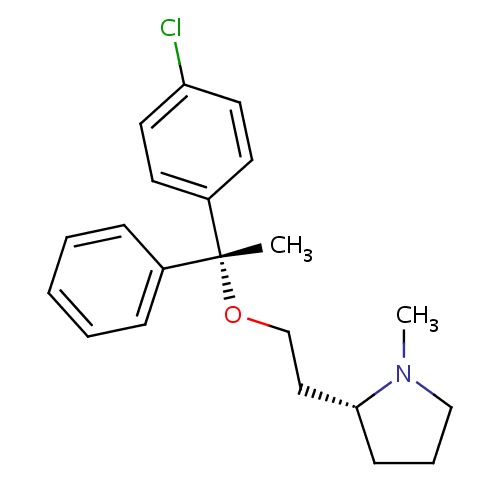
| FDBD00249 | Doxylamine |
|
Histamine H1 Antagonists; Antiemetics; Respiratory System; Aminoalkyl Ethers; Antihistamines for Systemic Use; | Used alone as a short-term sleep aid, in combination with other drugs as a night-time cold and allergy relief drug. Also used in combination with Vitamin B6 (pyridoxine) to prevent morning sickness in pregnant women. |
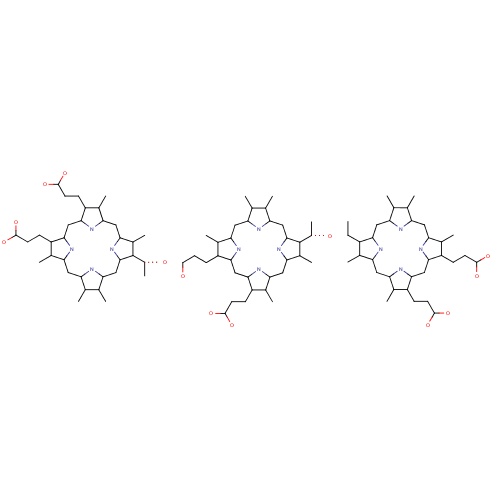
| FDBD00571 | Porfimer |
|
Antineoplastic Agents; Dermatologic Agents; Photosensitizing Agents; Antineoplastic and Immunomodulating Agents; Sensitizers Used in Photodynamic/radiation Therapy; | Indicated in the treatment of esophageal cancer. |
| FDBD00793 | Salmeterol |
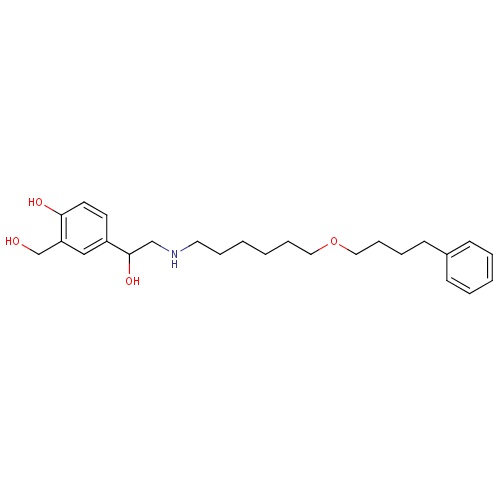
|
Sympathomimetics; Adrenergic beta-2 Receptor Agonists; Bronchodilator Agents; Respiratory System; Drugs for Obstructive Airway Diseases; Selective Beta-2-Adrenoreceptor Agonists; Adrenergics, Inhalants; Cytochrome P-450 CYP2C8 Inhibitors; Cytochrome P-450 CYP2C8 Inducers; CYP3A4 Inhibitors; Beta2 Agonists; | For the treatment of asthma and chronic obstructive pulmonary disease (COPD). |
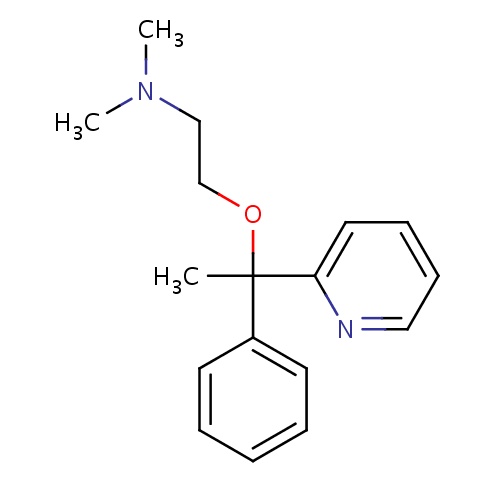
| FDBD00934 | Emedastine |

|
Anti-Allergic Agents; Histamine H1 Antagonists; Ophthalmologicals; Sensory Organs; Decongestants and Antiallergics; | For the temporary relief of the signs and symptoms of allergic conjunctivitis. |
| FDBD01514 | Pipazethate |

|
Antitussive Agents; Cough and Cold Preparations; Respiratory System; | For the treatment of cough. |
| FDBD01610 | Eprazinone |

|
Mucolytics; Cough and Cold Preparations; Respiratory System; | |
| FDBD01665 | Vilanterol |
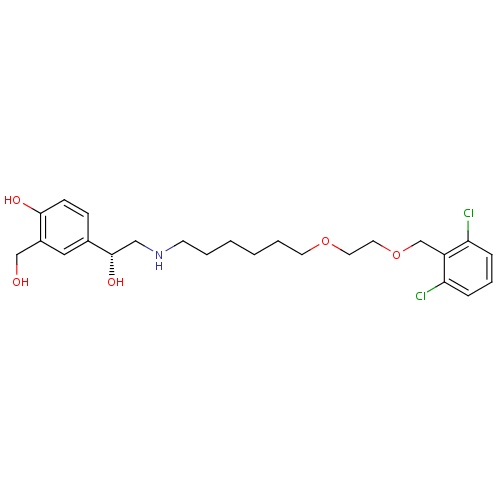
|
Immunosuppressive Agents; Respiratory System; Drugs for Obstructive Airway Diseases; Adrenergics, Inhalants; CYP3A4 Inhibitors; Beta2 Agonists; | Vilanterol is approved for use in several combination products such as with fluticasone furoate under the tradename Breo Ellipta and in combination with umeclidinium bromide as Anoro Ellipta. Approved by the FDA in 2013, use of Breo Ellipta is indicated for the long-term, once-daily maintenance treatment of airflow obstruction in patients with COPD, including chronic bronchitis and emphysema. It is also indicated for once-daily maintenance treatment of asthma in patients aged 18 or older with reversible obstructive airways disease. |
| FDBD01672 | Pinaverium |
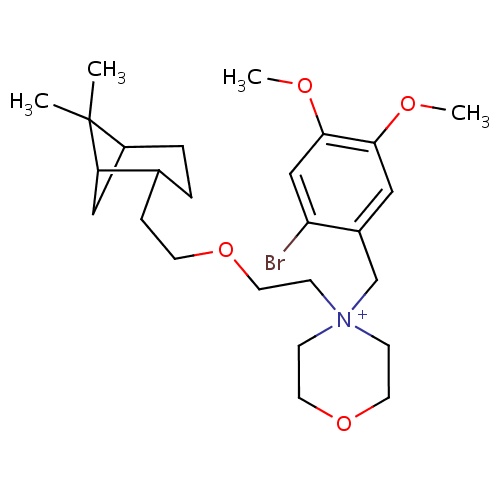
|
Alimentary Tract and Metabolism; Drugs for Functional Gastrointestinal Disorders; | |
| FDBD01715 | Florbetaben (18F) |
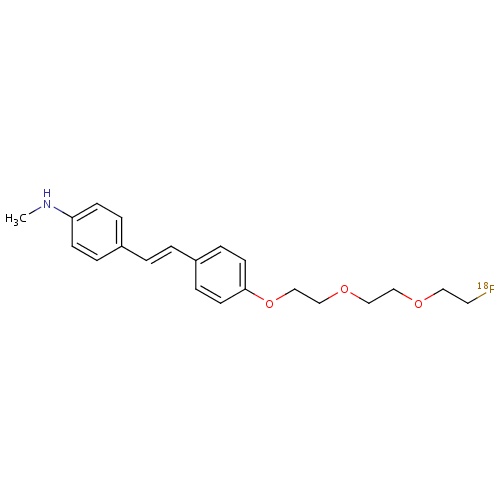
|
Diagnostic Radiopharmaceuticals; Central Nervous System; |
17 ,
2
| FRAGNAME | PDBID | SIMILIRITY | XSCORE | SMILE | HAC |
|---|---|---|---|---|---|
| 1ikt_ligand_4_645.mol2 | 1ikt | 1 | -5.98 | CCOCC | 5 |
| 1ikt_ligand_4_710.mol2 | 1ikt | 1 | -5.84 | CCOCC | 5 |
| 4q1n_ligand_3_163.mol2 | 4q1n | 1 | -5.56 | CCOCC | 5 |
| 3ggj_ligand_4_10.mol2 | 3ggj | 1 | -5.50 | C(OCC)C | 5 |
| 2bvs_ligand_4_1785.mol2 | 2bvs | 1 | -5.49 | CCOCC | 5 |
| 2xei_ligand_4_3045.mol2 | 2xei | 1 | -5.37 | CCOCC | 5 |
218 ,
22

