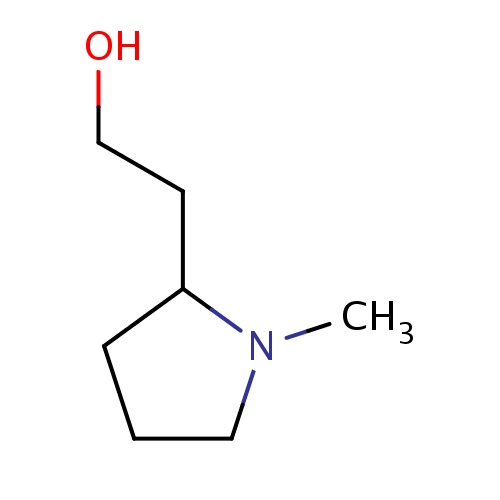
Common name
2-[(2R)-1-methylpyrrolidin-2-yl]ethanol
IUPAC name
2-[(2R)-1-methylpyrrolidin-2-yl]ethanol
SMILES
OCCC1N(CCC1)C
Common name
2-[(2R)-1-methylpyrrolidin-2-yl]ethanol
IUPAC name
2-[(2R)-1-methylpyrrolidin-2-yl]ethanol
SMILES
OCCC1N(CCC1)C
INCHI
InChI=1S/C7H15NO/c1-8-5-2-3-7(8)4-6-9/h7,9H,2-6H2,1H3/t7-/m1/s1
FORMULA
C7H15NO

Common name
2-[(2R)-1-methylpyrrolidin-2-yl]ethanol
IUPAC name
2-[(2R)-1-methylpyrrolidin-2-yl]ethanol
Molecular weight
129.200
clogP
0.806
clogS
-0.678
Frequency
0.0003
HBond Acceptor
2
HBond Donor
1
Total PolarSurface Area
23.47
Number of Rings
1
Rotatable Bond
2
| Drug ID | Common name | Structure CAS | Compound class | Therapeutic area |
|---|---|---|---|---|
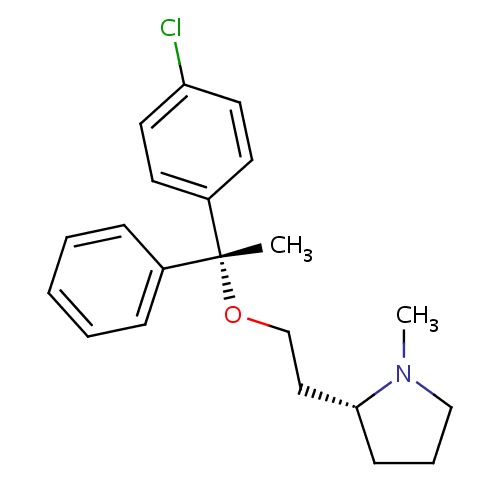
| FDBD00170 | Clemastine |
|
Anti-Allergic Agents; Antipruritics; Histamine H1 Antagonists; Respiratory System; Dermatologicals; Antipruritics, Incl. Antihistamines, Anesthetics, Etc.; Antihistamines for Topical Use; Aminoalkyl Ethers; Antihistamines for Systemic Use; CYP2D6 Inducers; CYP2D6 Inducers (strong); CYP3A4 Inhibitors; | For the relief of symptoms associated with allergic rhinitis such as sneezing, rhinorrhea, pruritus and acrimation. Also for the management of mild, uncomplicated allergic skin manifestations of urticaria and angioedema. Used as self-medication for temporary relief of symptoms associated with the common cold. |
1 ,
1
| FRAGNAME | PDBID | SIMILIRITY | XSCORE | SMILE | HAC |
|---|---|---|---|---|---|
| 2brp_ligand_6_165.mol2 | 2brp | 0.878788 | -7.45 | CCC[N@@H+]1[C@@H](C[C@H]2CC[C@H](C[C@H]12)O)[C@H]1CC[C@@H](CC1)O | 20 |
| 2brp_ligand_5_385.mol2 | 2brp | 0.878788 | -7.43 | O[C@@H]1CC[C@@H]2C[C@H]([N@@H+](CCC)[C@H]2C1)C1CCCCC1 | 19 |
| 2brp_ligand_5_55.mol2 | 2brp | 0.878788 | -7.32 | O[C@@H]1CC[C@@H]2C[C@H]([N@@H+](CC)[C@H]2C1)[C@H]1CC[C@@H](CC1)O | 19 |
| 2brp_ligand_4_121.mol2 | 2brp | 0.878788 | -7.30 | O[C@@H]1CC[C@@H]2C[C@H]([N@@H+](CC)[C@H]2C1)C1CCCCC1 | 18 |
| 2brp_ligand_4_11.mol2 | 2brp | 0.878788 | -7.08 | O[C@@H]1CC[C@@H]2C[C@H]([N@@H+](C)[C@H]2C1)[C@H]1CC[C@@H](CC1)O | 18 |
| 2brp_ligand_3_23.mol2 | 2brp | 0.878788 | -7.07 | O[C@@H]1CC[C@@H]2C[C@H]([N@@H+](C)[C@H]2C1)C1CCCCC1 | 17 |
| 2brp_ligand_2_3.mol2 | 2brp | 0.878788 | -6.96 | O[C@@H]1CC[C@@H]2C[C@H]([NH2+][C@H]2C1)C1CCCCC1 | 16 |
| 2iog_ligand_2_41.mol2 | 2iog | 0.878788 | -6.95 | O[C@@H]1C[C@@H]2[NH2+]C[C@H](C)[C@H]2CC1 | 11 |
| 2brp_ligand_5_1540.mol2 | 2brp | 0.878788 | -6.83 | O[C@@H]1CC[C@@H]2CC[N@@H+](CCCC)[C@H]2C1 | 14 |
| 2brp_ligand_4_671.mol2 | 2brp | 0.878788 | -6.72 | O[C@@H]1CC[C@@H]2CC[N@@H+](CCC)[C@H]2C1 | 13 |
113 ,
12

