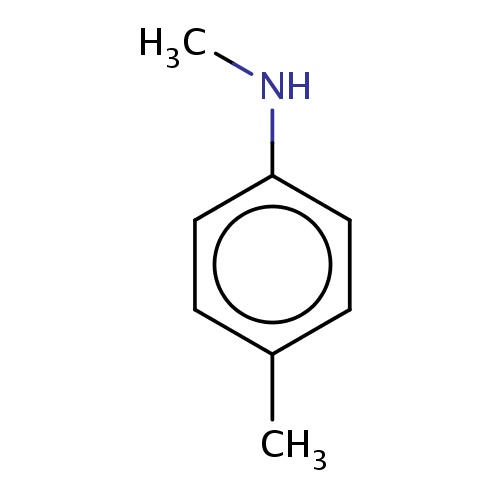
Common name
N,4-dimethylaniline
IUPAC name
N,4-dimethylaniline
SMILES
CNc1ccc(cc1)C
Common name
N,4-dimethylaniline
IUPAC name
N,4-dimethylaniline
SMILES
CNc1ccc(cc1)C
INCHI
InChI=1S/C8H11N/c1-7-3-5-8(9-2)6-4-7/h3-6,9H,1-2H3
FORMULA
C8H11N

Common name
N,4-dimethylaniline
IUPAC name
N,4-dimethylaniline
Molecular weight
121.180
clogP
1.992
clogS
-2.570
Frequency
0.0010
HBond Acceptor
0
HBond Donor
1
Total PolarSurface Area
12.03
Number of Rings
1
Rotatable Bond
1
| Drug ID | Common name | Structure CAS | Compound class | Therapeutic area |
|---|---|---|---|---|
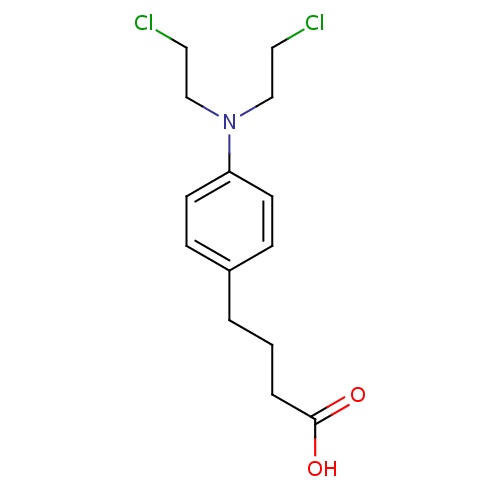
| FDBD00178 | Chlorambucil |
|
Antineoplastic Agents; Immunosuppressive Agents; Antineoplastic Agents, Alkylating; Alkylating Agents; Antineoplastic and Immunomodulating Agents; Nitrogen Mustard Analogues; | For treatment of chronic lymphatic (lymphocytic) leukemia, childhood minimal-change nephrotic syndrome, and malignant lymphomas including lymphosarcoma, giant follicular lymphoma, Hodgkin's disease, non-Hodgkin's lymphomas, and Waldenström's Macroglobulinemia. |
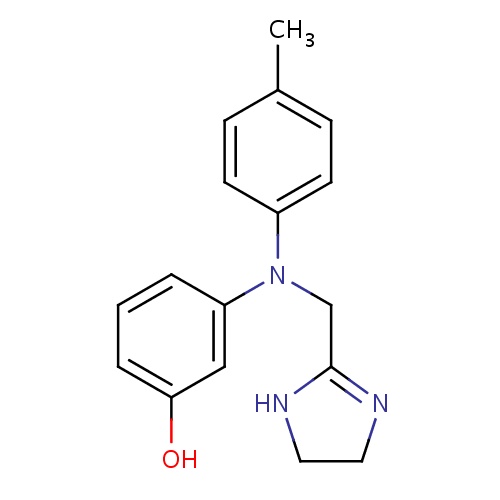
| FDBD00557 | Phentolamine |
|
Antihypertensive Agents; Adrenergic alpha-1 Receptor Antagonists; Adrenergic alpha-Antagonists; Antidotes; Cardiovascular System; Peripheral Vasodilators; Imidazoline Derivatives; | Used as an aid for the diagnosis of pheochromocytoma, and may be administered immediately prior to or during pheochromocytomectomy to prevent or control paroxysmal hypertension resulting from anesthesia, stress, or operative manipulation of the tumor. Phentolamine has also been used to treat hypertensive crisis caused by sympathomimetic amines or catecholamine excess by certain foods or drugs in patients taking MAO inhibitors, or by clonidine withdrawal syndrome. Other indications include the prevention of dermal necrosis and sloughing following IV administration or extravasation of norepinephrine, decrease in impedance to left ventricular ejection and the infarct size in patients with MI associated with left ventricular failure, treatment of erectile dysfunction through self-injection of small doses combined with papaverine hydrochloride into the corpus cavernosum, and as an adjunct to the management of cocaine overdose to reverse coronary vasoconstriction following use of oxygen, benzodiazepines,and nitroglycerin. |
| FDBD00894 | Melphalan |

|
Antineoplastic Agents; Immunosuppressive Agents; Antineoplastic Agents, Alkylating; Alkylating Agents; Myeloablative Agonists; Antineoplastic and Immunomodulating Agents; Nitrogen Mustard Analogues; | For the palliative treatment of multiple myeloma and for the palliation of non-resectable epithelial carcinoma of the ovary. Has also been used alone or as part of various chemotherapeutic regimens as an adjunct to surgery in the treatment of breast cancer, alone or in combination regimens for palliative treatment of locally recurrent or unresectable in-transit metastatic melanoma of the extremities, as well as for the treatment of amyloidosis with prednisone. |
3 ,
1
| FRAGNAME | PDBID | SIMILIRITY | XSCORE | SMILE | HAC |
|---|---|---|---|---|---|
| 4k3h_ligand_2_5.mol2 | 4k3h | 1 | -7.28 | c1(ccc(cc1)N(C)C)C | 10 |
| 4k3h_ligand_2_8.mol2 | 4k3h | 1 | -7.25 | c1(ccc(cc1)N(C)C)C | 10 |
| 4hj2_ligand_4_2346.mol2 | 4hj2 | 1 | -6.68 | CN(C)c1ccc(cc1)C | 10 |
| 1lpg_ligand_2_22.mol2 | 1lpg | 1 | -6.63 | Cc1ccc(cc1)[N](C)(C)C | 11 |
| 4hj2_ligand_3_651.mol2 | 4hj2 | 1 | -6.62 | CNc1ccc(cc1)C | 9 |
| 4hj2_ligand_3_661.mol2 | 4hj2 | 1 | -6.59 | CNc1ccc(cc1)C | 9 |
| 3btc_ligand_2_8.mol2 | 3btc | 1 | -6.58 | N(C)(C)c1ccc(C)cc1 | 10 |
100 ,
11

