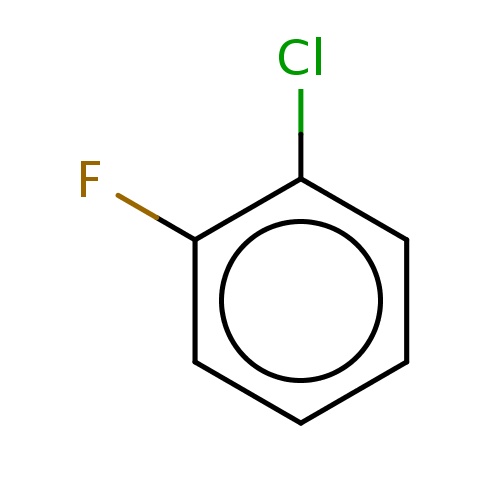
Common name
1-chloro-2-fluoro-benzene
IUPAC name
1-chloro-2-fluoro-benzene
SMILES
Clc1ccccc1F
Common name
1-chloro-2-fluoro-benzene
IUPAC name
1-chloro-2-fluoro-benzene
SMILES
Clc1ccccc1F
INCHI
InChI=1S/C6H4ClF/c7-5-3-1-2-4-6(5)8/h1-4H
FORMULA
C6H4ClF

Common name
1-chloro-2-fluoro-benzene
IUPAC name
1-chloro-2-fluoro-benzene
Molecular weight
130.547
clogP
2.962
clogS
-2.650
Frequency
0.0021
HBond Acceptor
0
HBond Donor
0
Total PolarSurface Area
0
Number of Rings
1
Rotatable Bond
0
| Drug ID | Common name | Structure CAS | Compound class | Therapeutic area |
|---|---|---|---|---|
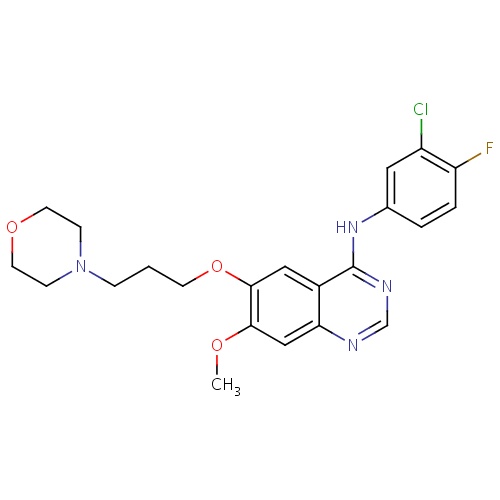
| FDBD00203 | Gefitinib |
|
Antineoplastic Agents; Protein Kinase Inhibitors; Antineoplastic and Immunomodulating Agents; Cytochrome P-450 CYP2C9 Inhibitors; Cytochrome P-450 CYP2C9 Inducers; Cytochrome P-450 CYP2C19 Inducers; CYP2D6 Inducers; CYP2D6 Inducers (strong); CYP3A4 Inhibitors; | For the continued treatment of patients with locally advanced or metastatic non-small cell lung cancer after failure of either platinum-based or docetaxel chemotherapies. |
| FDBD01575 | Afatinib |

|
Antineoplastic Agents; Protein Kinase Inhibitors; Antineoplastic and Immunomodulating Agents; | Afatinib is a kinase inhibitor indicated for the first-line treatment of patient with metastatic non-small cell lung cancer (NSCLC) whose tumours have epidermal growth factor receptor (EGFR) exon 19 deletions or exon 21 (L858R) substitution mutations as detected by an FDA-approved test. |
| FDBD01681 | Elvitegravir |

|
Antiinfectives for Systemic Use; Direct Acting Antivirals; Antivirals for Systemic Use; CYP3A4 Inhibitors; | Elvitegravir in combination with an HIV protease inhibitor coadministered with ritonavir and with other antiretroviral drug(s) is indicated for the treatment of HIV-1 infection in antiretroviral treatment-experienced adults. |
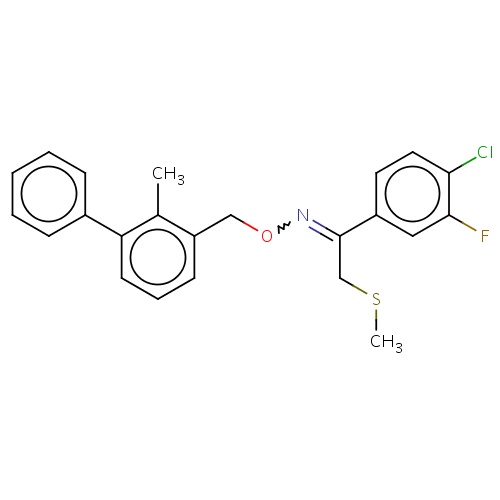
| FDBD02310 | thiofluoximate |
|
Insecticide | Insecticide |
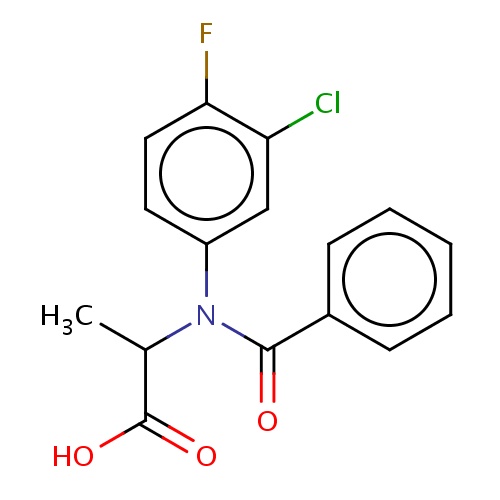
| FDBD02404 | flamprop |
|
Herbicide | Herbicide |
| FDBD02405 | flamprop-M |
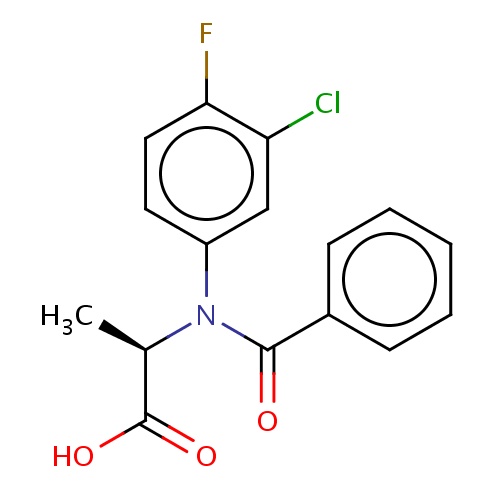
|
Herbicide | Herbicide |
6 ,
1
| FRAGNAME | PDBID | SIMILIRITY | XSCORE | SMILE | HAC |
|---|---|---|---|---|---|
| 4i54_ligand_frag_4.mol2 | 4i54 | 1 | -7.02 | c1cc(F)c(Cl)cc1 | 8 |
| 4dkp_ligand_frag_1.mol2 | 4dkp | 1 | -7.01 | c1cc(c(cc1)Cl)F | 8 |
| 4dkq_ligand_frag_1.mol2 | 4dkq | 1 | -7.01 | c1cc(c(cc1)Cl)F | 8 |
| 4dko_ligand_frag_2.mol2 | 4dko | 1 | -6.98 | c1c(c(ccc1)F)Cl | 8 |
| 4dkr_ligand_frag_2.mol2 | 4dkr | 1 | -6.94 | c1c(c(ccc1)F)Cl | 8 |
| 3b5r_ligand_frag_0.mol2 | 3b5r | 1 | -6.87 | c1c(F)c(Cl)ccc1 | 8 |
| 3d5m_ligand_frag_0.mol2 | 3d5m | 1 | -6.72 | c1cc(c(cc1)Cl)F | 8 |
| 2qe2_ligand_frag_1.mol2 | 2qe2 | 1 | -6.59 | c1cc(c(cc1)Cl)F | 8 |
| 2itz_ligand_frag_8.mol2 | 2itz | 1 | -6.55 | c1cc(Cl)c(F)cc1 | 8 |
| 2ito_ligand_frag_8.mol2 | 2ito | 1 | -6.53 | c1cc(Cl)c(F)cc1 | 8 |
1095 ,
110

