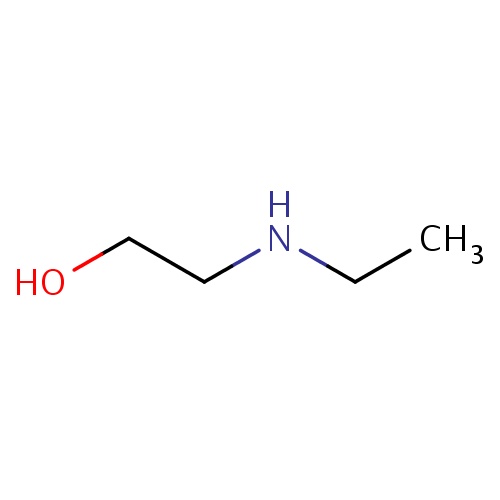
Common name
2-(ethylamino)ethanol
IUPAC name
2-(ethylamino)ethanol
SMILES
N(CCO)CC
Common name
2-(ethylamino)ethanol
IUPAC name
2-(ethylamino)ethanol
SMILES
N(CCO)CC
INCHI
InChI=1S/C4H11NO/c1-2-5-3-4-6/h5-6H,2-4H2,1H3
FORMULA
C4H11NO

Common name
2-(ethylamino)ethanol
IUPAC name
2-(ethylamino)ethanol
Molecular weight
89.136
clogP
-0.191
clogS
-0.859
Frequency
0.0065
HBond Acceptor
1
HBond Donor
2
Total PolarSurface Area
32.26
Number of Rings
0
Rotatable Bond
3
| Drug ID | Common name | Structure CAS | Compound class | Therapeutic area |
|---|---|---|---|---|
| FDBD01111 | Arformoterol |

|
Adrenergic beta-2 Receptor Agonists; Bronchodilator Agents; Cytochrome P-450 CYP2C9 Inhibitors; Cytochrome P-450 CYP2C9 Inducers; Cytochrome P-450 CYP2C19 Inducers; CYP2A6 Inhibitors; CYP2A6 Inhibitors (strong); CYP2A6 Inhibitors (moderate); CYP2A6 Inducers; CYP2A6 Inducers (strong); CYP2D6 Inducers; CYP2D6 Inducers (strong); Beta2 Agonists; | A bronchodilator used for the long term, symptomatic treatment of reversible bronchoconstriction in patients with chronic obstructive pulmonary disease (COPD), including chronic bronchitis and emphysema. |
| FDBD01116 | Fenoterol |

|
Sympathomimetics; Adrenergic beta-2 Receptor Agonists; Bronchodilator Agents; Tocolytic Agents; Respiratory System; Drugs for Obstructive Airway Diseases; Genito Urinary System and Sex Hormones; Selective Beta-2-Adrenoreceptor Agonists; Adrenergics, Inhalants; Adrenergics for Systemic Use; Sympathomimetics, Labour Repressants; Beta2 Agonists; | Fenoterol is used for the treatment of asthma. |
| FDBD01120 | Bevantolol |
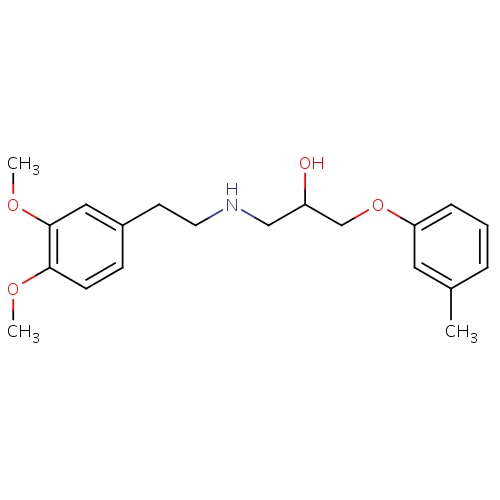
|
Adrenergic beta-1 Receptor Antagonists; Adrenergic alpha-1 Receptor Antagonists; Cardiovascular System; Beta Blocking Agents, Selective; Beta Blocking Agents; Beta Blocking Agents, Selective, and Thiazides; Beta Blocking Agents and Thiazides; | For the treatment of angina pectoris and hypertension. |
| FDBD01261 | Hydroxychloroquine |
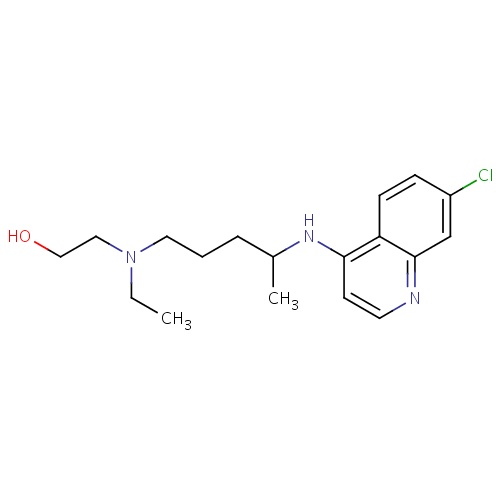
|
Antirheumatic Agents; Enzyme Inhibitors; Antimalarials; Antiprotozoal Agents; Antiparasitic Products, Insecticides and Repellents; Aminoquinolines; CYP2D6 Inducers; CYP2D6 Inducers (strong); | For the suppressive treatment and treatment of acute attacks of malaria due to . |
| FDBD01385 | Silodosin |
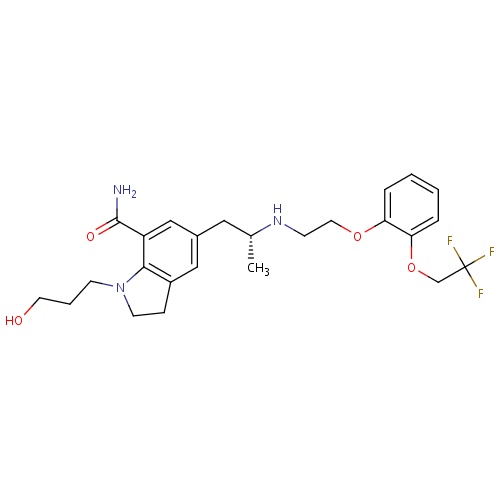
|
Adrenergic alpha-1 Receptor Antagonists; Adrenergic alpha-Antagonists; Genito Urinary System and Sex Hormones; Drugs Used in Benign Prostatic Hypertrophy; Urological Agents; CYP3A4 Inhibitors; | Treatment for symptomatic relief of benign prostatic hyperplasia . |
| FDBD01448 | Lumefantrine |
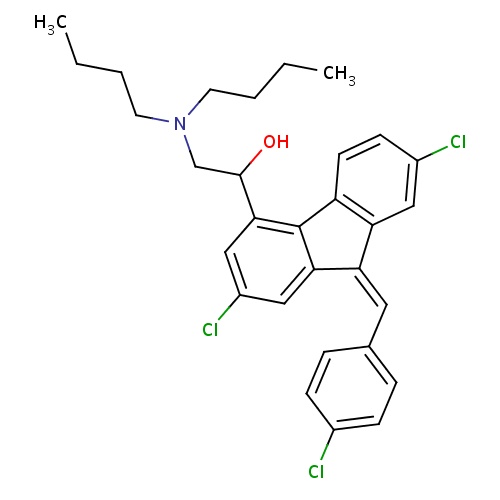
|
Antimalarials; Antiprotozoal Agents; Antiparasitic Products, Insecticides and Repellents; CYP2D6 Inducers; CYP2D6 Inducers (strong); CYP3A4 Inhibitors; | Lumefantrine and artemether combination therapy is indicated for the treatment of acute uncomplicated malaria caused by . |
| FDBD01559 | Mirabegron |
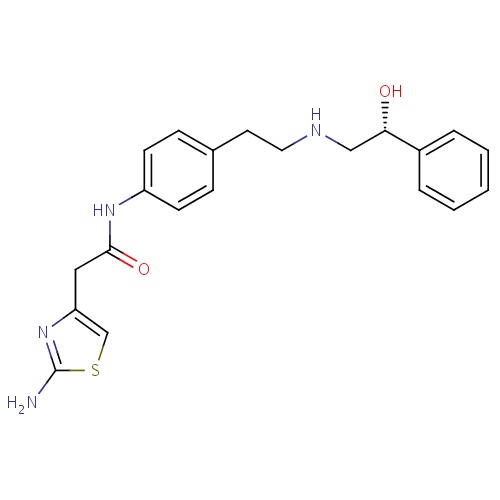
|
Muscle Relaxants, Genitourinary; Genito Urinary System and Sex Hormones; Drugs for Urinary Frequency and Incontinence; Urological Agents; CYP2D6 Inducers; CYP2D6 Inducers (strong); CYP3A4 Inhibitors; Adrenergic beta-3 Receptor Agonists; | Mirabegron is a beta-3 adrenergic agonist indicated for the treatment of overactive bladder (OAB) with symptoms of urge urinary incontinence, urgency, and urinary frequency. |
| FDBD01592 | Hexoprenaline |
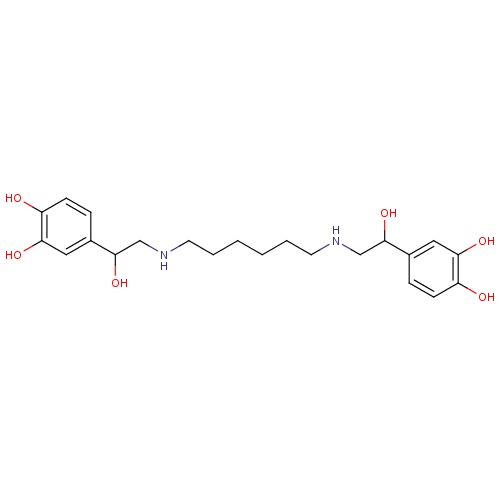
|
Adrenergic beta-2 Receptor Agonists; Bronchodilator Agents; Tocolytic Agents; Respiratory System; Drugs for Obstructive Airway Diseases; Selective Beta-2-Adrenoreceptor Agonists; Adrenergics, Inhalants; Adrenergics for Systemic Use; | |
| FDBD01665 | Vilanterol |
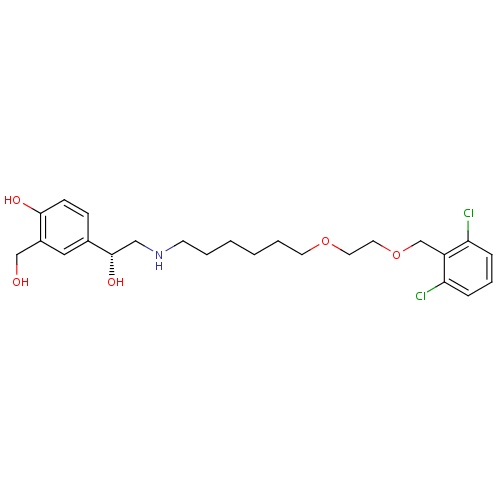
|
Immunosuppressive Agents; Respiratory System; Drugs for Obstructive Airway Diseases; Adrenergics, Inhalants; CYP3A4 Inhibitors; Beta2 Agonists; | Vilanterol is approved for use in several combination products such as with fluticasone furoate under the tradename Breo Ellipta and in combination with umeclidinium bromide as Anoro Ellipta. Approved by the FDA in 2013, use of Breo Ellipta is indicated for the long-term, once-daily maintenance treatment of airflow obstruction in patients with COPD, including chronic bronchitis and emphysema. It is also indicated for once-daily maintenance treatment of asthma in patients aged 18 or older with reversible obstructive airways disease. |
19 ,
2
| FRAGNAME | PDBID | SIMILIRITY | XSCORE | SMILE | HAC |
|---|---|---|---|---|---|
| 3c2u_ligand_3_37.mol2 | 3c2u | 1 | -5.98 | CC[NH2+]CCO | 6 |
| 3c2u_ligand_3_36.mol2 | 3c2u | 1 | -5.89 | CC[NH2+]CCO | 6 |
| 2xfk_ligand_2_68.mol2 | 2xfk | 1 | -5.83 | C(C)[NH2+]CCO | 6 |
| 2viy_ligand_2_49.mol2 | 2viy | 1 | -5.82 | C([NH2+]CC)CO | 6 |
| 2xfi_ligand_2_10.mol2 | 2xfi | 1 | -5.82 | C([NH2+]CC)CO | 6 |
| 2viz_ligand_2_63.mol2 | 2viz | 1 | -5.81 | C(O)C[NH2+]CC | 6 |
| 2vj6_ligand_2_56.mol2 | 2vj6 | 1 | -5.80 | C(O)C[NH2+]CC | 6 |
| 2xfj_ligand_2_56.mol2 | 2xfj | 1 | -5.79 | C([NH2+]CC)CO | 6 |
| 4qkx_ligand_3_28.mol2 | 4qkx | 1 | -5.75 | CC[NH2+]CCO | 6 |
| 3c2u_ligand_3_38.mol2 | 3c2u | 1 | -5.70 | CC[NH2+]CCO | 6 |
195 ,
20

