
Common name
(1S,5R)-8-isopropyl-8-methyl-8λ4-azabicyclo[3.2.1]octane
IUPAC name
(1S,5R)-8-isopropyl-8-methyl-8λ4-azabicyclo[3.2.1]octane
SMILES
CC(C)[N]1(C2CCC1CCC2)C
Common name
(1S,5R)-8-isopropyl-8-methyl-8λ4-azabicyclo[3.2.1]octane
IUPAC name
(1S,5R)-8-isopropyl-8-methyl-8λ4-azabicyclo[3.2.1]octane
SMILES
CC(C)[N]1(C2CCC1CCC2)C
INCHI
InChI=1S/C11H22N/c1-9(2)12(3)10-5-4-6-11(12)8-7-10/h9-11H,4-8H2,1-3H3/t10-,11+
FORMULA
C11H22N

Common name
(1S,5R)-8-isopropyl-8-methyl-8λ4-azabicyclo[3.2.1]octane
IUPAC name
(1S,5R)-8-isopropyl-8-methyl-8λ4-azabicyclo[3.2.1]octane
Molecular weight
168.299
clogP
-0.432
clogS
-2.616
Frequency
0.0003
HBond Acceptor
0
HBond Donor
0
Total Polar
Surface Area
0
Number of Rings
2
Rotatable Bond
1
| Drug ID | Common name | Structure CAS | Compound class | Therapeutic area |
|---|---|---|---|---|
| FDBD00217 | Ipratropium bromide |
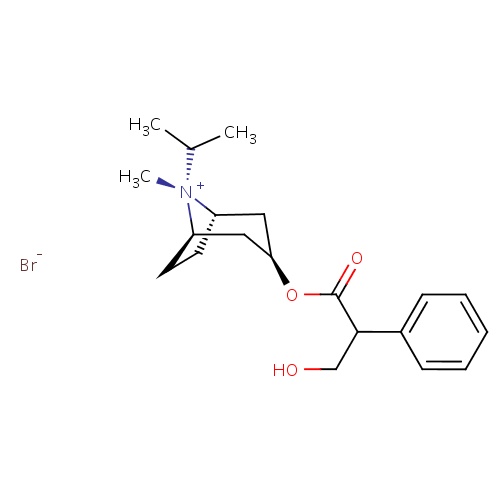
|
Bronchodilator Agents; Muscarinic Antagonists; Cholinergic Antagonists; Antispasmodics; Respiratory System; Drugs for Obstructive Airway Diseases; Nasal Preparations; Adrenergics, Inhalants; Anticholinergics; CYP2D6 Inducers; CYP2D6 Inducers (strong); CYP3A4 Inhibitors; | For maintenance treatment of bronchospasm associated with chronic obstructive pulmonary disease, including chronic bronchitis and emphysema. |
1 ,
1
| FRAGNAME | PDBID | SIMILIRITY | XSCORE | SMILE | HAC |
|---|---|---|---|---|---|
| 2y57_ligand_2_3.mol2 | 2y57 | 1 | -7.48 | CC[N@@H+]1[C@H]2CCC[C@@H]1CC2 | 10 |
| 2y57_ligand_1_1.mol2 | 2y57 | 1 | -7.13 | C[N@@H+]1[C@H]2CCC[C@@H]1CC2 | 9 |
| 2wnc_ligand_frag_1.mol2 | 2wnc | 1 | -7.08 | C1C[C@H]2[N@H+]([C@@H]1CCC2)C | 9 |
| 2y58_ligand_1_1.mol2 | 2y58 | 1 | -7.08 | C1C[C@H]2CC[C@@H](C1)[N+]2(C)C | 10 |
| 2y58_ligand_frag_1.mol2 | 2y58 | 1 | -6.96 | C1C[C@H]2CC[C@@H](C1)[N@@H+]2C | 9 |
| 2y56_ligand_1_1.mol2 | 2y56 | 1 | -6.88 | C[N@H+]1[C@@H]2CCC[C@H]1CC2 | 9 |
| 4dbm_ligand_frag_1.mol2 | 4dbm | 1 | -6.60 | C1C[C@H]2CC[C@H]([N+]2(C)C)C1 | 10 |
| 2xzc_ligand_frag_1.mol2 | 2xzc | 1 | -6.50 | C1C[C@@H]2[N@H+]([C@@H](CC2)C1)C | 9 |
| 2w8g_ligand_1_2.mol2 | 2w8g | 1 | -6.49 | C1C[C@@H]2CC[C@H](C1)[N@H+]2C | 9 |
| 2qhm_ligand_frag_1.mol2 | 2qhm | 1 | -6.15 | C1C[C@H]2[N@H+]([C@@H]1CCC2)C | 9 |
562 ,
57

