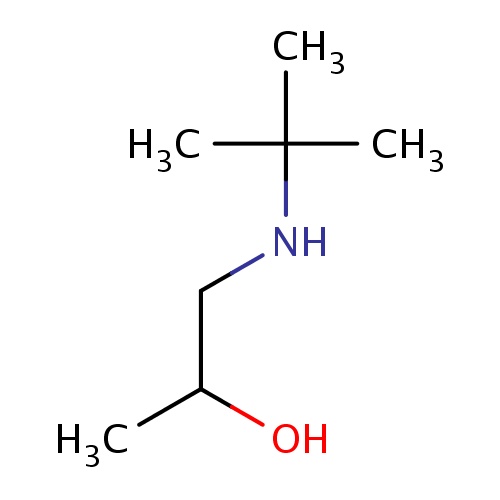
Common name
(2S)-1-(tert-butylamino)propan-2-ol
IUPAC name
(2S)-1-(tert-butylamino)propan-2-ol
SMILES
C(C(C)O)NC(C)(C)C
Common name
(2S)-1-(tert-butylamino)propan-2-ol
IUPAC name
(2S)-1-(tert-butylamino)propan-2-ol
SMILES
C(C(C)O)NC(C)(C)C
INCHI
InChI=1S/C7H17NO/c1-6(9)5-8-7(2,3)4/h6,8-9H,5H2,1-4H3/t6-/m0/s1
FORMULA
C7H17NO

Common name
(2S)-1-(tert-butylamino)propan-2-ol
IUPAC name
(2S)-1-(tert-butylamino)propan-2-ol
Molecular weight
131.216
clogP
0.527
clogS
-1.371
Frequency
0.0010
HBond Acceptor
1
HBond Donor
2
Total PolarSurface Area
32.26
Number of Rings
0
Rotatable Bond
3
| Drug ID | Common name | Structure CAS | Compound class | Therapeutic area |
|---|---|---|---|---|
| FDBD00255 | Timolol |
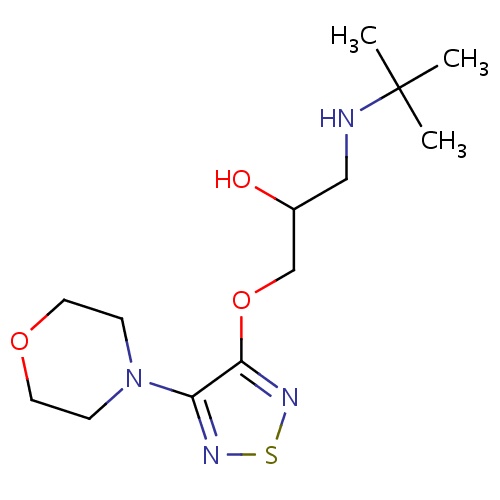
|
Antihypertensive Agents; Anti-Arrhythmia Agents; Adrenergic beta-Antagonists; Ophthalmologicals; Sensory Organs; Cardiovascular System; Beta Blocking Agents; Antiglaucoma Preparations and Miotics; Beta Blocking Agents and Thiazides; Beta Blocking Agents, Non-Selective; Beta Blocking Agents, Non-Selective, and Thiazides; Cytochrome P-450 CYP2C19 Inducers; CYP2D6 Inducers; CYP2D6 Inducers (strong); | In its oral form it is used to treat high blood pressure and prevent heart attacks, and occasionally to prevent migraine headaches. In its opthalmic form it is used to treat open-angle and occasionally secondary glaucoma. |
| FDBD01331 | Celiprolol |
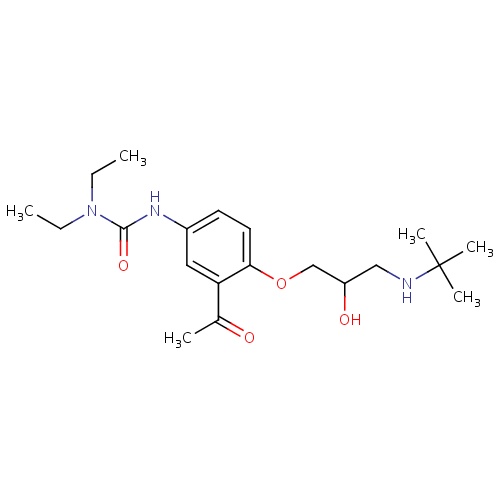
|
Antihypertensive Agents; Sympathomimetics; Adrenergic beta-1 Receptor Antagonists; Anti-Arrhythmia Agents; Vasodilator Agents; Cardiovascular System; Beta Blocking Agents, Selective; Beta Blocking Agents; CYP3A4 Inhibitors; Beta2 Agonists; | Celiprolol is indicated for the management of mild to moderate hypertension and effort-induced angina pectoris. |
| FDBD01737 | Arotinolol |
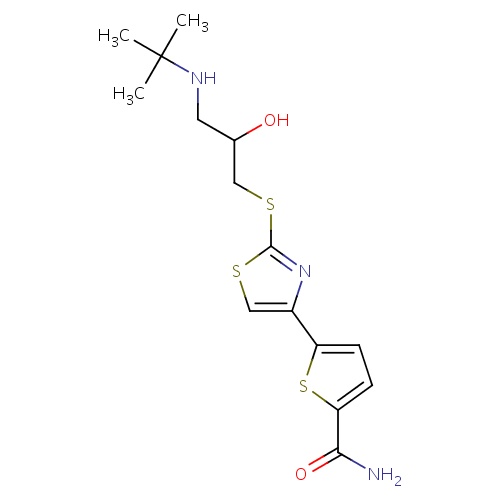
|
; | Used in the treatment of high blood pressure. |
3 ,
1
| FRAGNAME | PDBID | SIMILIRITY | XSCORE | SMILE | HAC |
|---|---|---|---|---|---|
| 2vie_ligand_4_573.mol2 | 2vie | 1 | -6.86 | [C@H](O)(C)C[NH2+]C(C)(C)C | 9 |
| 1dy4_ligand_3_9.mol2 | 1dy4 | 1 | -6.81 | C[C@@H](O)C[NH2+]C(C)C | 8 |
| 2vie_ligand_3_157.mol2 | 2vie | 1 | -6.48 | C(C)(C)[NH2+]C[C@@H](O)C | 8 |
| 1h46_ligand_3_9.mol2 | 1h46 | 1 | -6.47 | [C@H](O)(C)C[NH2+]C(C)C | 8 |
| 2xfk_ligand_3_256.mol2 | 2xfk | 1 | -6.24 | C(C)[NH2+]C[C@@H](O)C | 7 |
| 2viy_ligand_3_157.mol2 | 2viy | 1 | -6.23 | C([NH2+]CC)[C@@H](O)C | 7 |
| 2xfi_ligand_3_45.mol2 | 2xfi | 1 | -6.23 | C([NH2+]CC)[C@H](C)O | 7 |
| 4ya8_ligand_3_357.mol2 | 4ya8 | 1 | -6.23 | C[C@@H](O)C[NH2+]C(C)C | 8 |
| 2viz_ligand_3_236.mol2 | 2viz | 1 | -6.21 | C[C@H](O)C[NH2+]CC | 7 |
| 2vj6_ligand_3_190.mol2 | 2vj6 | 1 | -6.21 | C[C@H](O)C[NH2+]CC | 7 |
151 ,
16

