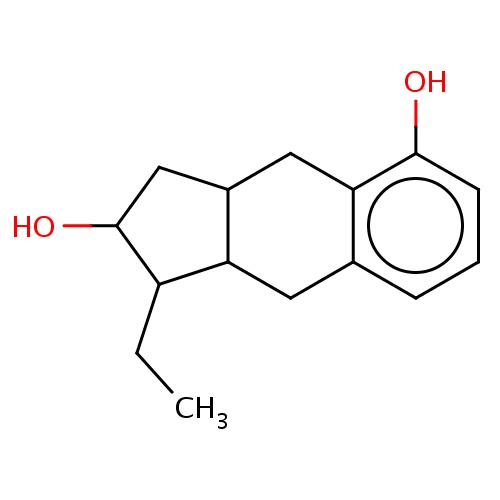
Common name
(1R,2R,3aS,9aS)-1-ethyl-2,3,3a,4,9,9a-hexahydro-1H-cyclopenta[g]naphthalene-2,5-diol
IUPAC name
(1R,2R,3aS,9aS)-1-ethyl-2,3,3a,4,9,9a-hexahydro-1H-cyclopenta[g]naphthalene-2,5-diol
SMILES
Oc1c2c(ccc1)CC3C(C2)CC(C3CC)O
Common name
(1R,2R,3aS,9aS)-1-ethyl-2,3,3a,4,9,9a-hexahydro-1H-cyclopenta[g]naphthalene-2,5-diol
IUPAC name
(1R,2R,3aS,9aS)-1-ethyl-2,3,3a,4,9,9a-hexahydro-1H-cyclopenta[g]naphthalene-2,5-diol
SMILES
Oc1c2c(ccc1)CC3C(C2)CC(C3CC)O
INCHI
InChI=1S/C15H20O2/c1-2-11-12-6-9-4-3-5-14(16)13(9)7-10(12)8-15(11)17/h3-5,10-12,15-17H,2,6-8H2,1H3/t10-,11+,12-,15+/m0/s1
FORMULA
C15H20O2

Common name
(1R,2R,3aS,9aS)-1-ethyl-2,3,3a,4,9,9a-hexahydro-1H-cyclopenta[g]naphthalene-2,5-diol
IUPAC name
(1R,2R,3aS,9aS)-1-ethyl-2,3,3a,4,9,9a-hexahydro-1H-cyclopenta[g]naphthalene-2,5-diol
Molecular weight
232.318
clogP
2.661
clogS
-2.609
Frequency
0.0003
HBond Acceptor
2
HBond Donor
2
Total PolarSurface Area
40.46
Number of Rings
3
Rotatable Bond
1
| Drug ID | Common name | Structure CAS | Compound class | Therapeutic area |
|---|---|---|---|---|
| FDBD00256 | Treprostinil |
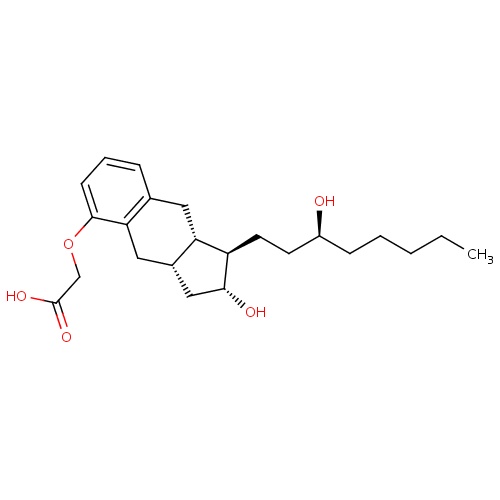
|
Antithrombins; Anticoagulants; Antihypertensive Agents; Antithrombotic Agents; Blood and Blood Forming Organs; Platelet Aggregation Inhibitors Excl. Heparin; Cytochrome P-450 CYP2C9 Inhibitors; Cytochrome P-450 CYP2C9 Inducers; | For use as a continuous subcutaneous infusion or intravenous infusion (for those not able to tolerate a subcutaneous infusion) for the treatment of pulmonary arterial hypertension in patients with NYHA Class II-IV symptoms to diminish symptoms associated with exercise. |
| FRAGNAME | PDBID | SIMILIRITY | XSCORE | SMILE | HAC |
|---|---|---|---|---|---|
| 1lhu_ligand.mol2 | 1lhu | 0.894737 | -10.22 | c1cc(O)cc2CC[C@@H]3[C@@H](c12)CC[C@]1([C@H]3CC[C@@H]1O)C | 21 |
| 1g50_ligand.mol2 | 1g50 | 0.894737 | -10.01 | c1cc(O)cc2CC[C@@H]3[C@@H](c12)CC[C@]1([C@H]3CC[C@@H]1O)C | 21 |
| 1jgl_ligand.mol2 | 1jgl | 0.894737 | -9.99 | c1cc(O)cc2CC[C@@H]3[C@@H](c12)CC[C@]1([C@H]3CC[C@@H]1O)C | 21 |
| 2j7x_ligand.mol2 | 2j7x | 0.894737 | -9.96 | c1cc(O)cc2CC[C@@H]3[C@@H](c12)CC[C@]1([C@H]3CC[C@@H]1O)C | 21 |
| 1a52_ligand.mol2 | 1a52 | 0.894737 | -9.84 | c1cc(O)cc2CC[C@@H]3[C@@H](c12)CC[C@]1([C@H]3CC[C@@H]1O)C | 21 |
| 1qku_ligand.mol2 | 1qku | 0.894737 | -9.81 | c1cc(O)cc2CC[C@@H]3[C@@H](c12)CC[C@]1([C@H]3CC[C@@H]1O)C | 21 |
| 1qkt_ligand.mol2 | 1qkt | 0.894737 | -9.64 | c1cc(O)cc2CC[C@@H]3[C@@H](c12)CC[C@]1([C@H]3CC[C@@H]1O)C | 21 |
| 2d06_ligand.mol2 | 2d06 | 0.894737 | -9.55 | c1cc(O)cc2CC[C@@H]3[C@@H](c12)CC[C@]1([C@H]3CC[C@@H]1O)C | 21 |
| 2bw7_ligand.mol2 | 2bw7 | 0.87931 | -7.91 | c1c2c(cc(c1O)O)CC[C@H]1[C@H]3[C@](CC[C@H]21)([C@H](CC3)O)C | 22 |
| 3h0a_ligand_1_7.mol2 | 3h0a | 0.854545 | -7.23 | c12CCCCc1cccc2O | 11 |

