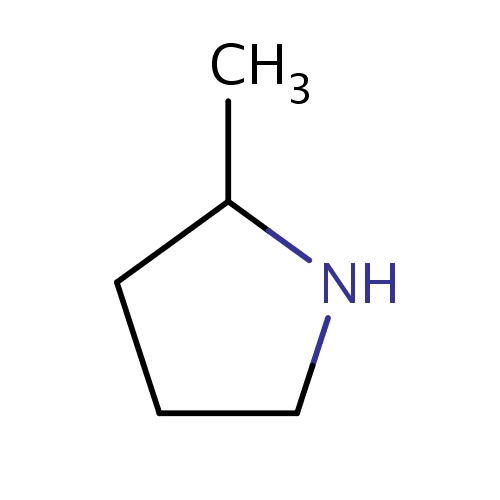
Common name
(2R)-2-methylpyrrolidine
IUPAC name
(2R)-2-methylpyrrolidine
SMILES
CC1NCCC1
Common name
(2R)-2-methylpyrrolidine
IUPAC name
(2R)-2-methylpyrrolidine
SMILES
CC1NCCC1
INCHI
InChI=1S/C5H11N/c1-5-3-2-4-6-5/h5-6H,2-4H2,1H3/t5-/m1/s1
FORMULA
C5H11N

Common name
(2R)-2-methylpyrrolidine
IUPAC name
(2R)-2-methylpyrrolidine
Molecular weight
85.148
clogP
1.392
clogS
-1.089
Frequency
0.0014
HBond Acceptor
0
HBond Donor
1
Total PolarSurface Area
12.03
Number of Rings
1
Rotatable Bond
0
| Drug ID | Common name | Structure CAS | Compound class | Therapeutic area |
|---|---|---|---|---|
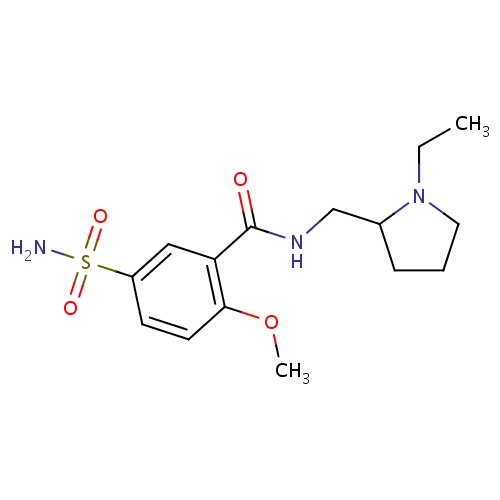
| FDBD00271 | Sulpiride |
|
Antidepressive Agents, Second-Generation; Antipsychotic Agents; Dopamine Antagonists; Antidepressive Agents; Nervous System; Psycholeptics; Benzamides; | Sulpiride is indicated for the treatment of schizophrenia. |
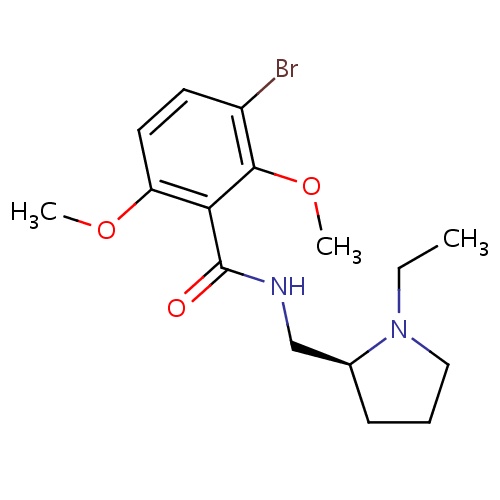
| FDBD00288 | Remoxipride |
|
Antipsychotic Agents; Dopamine Antagonists; Nervous System; Psycholeptics; Benzamides; CYP2D6 Inducers; CYP2D6 Inducers (strong); | Remoxipride is an atypical antipsychotic once used for the treatment of schizophrenia. |
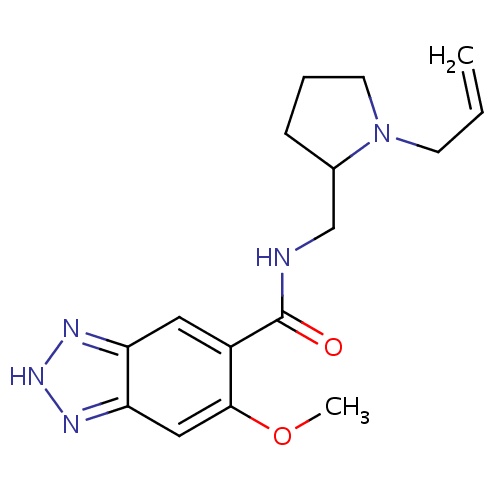
| FDBD01200 | Alizapride |
|
Antiemetics; Prokinetic Agents; Alimentary Tract and Metabolism; Drugs for Functional Gastrointestinal Disorders; Propulsives; | Alizapride is used in the treatment of nausea and vomiting, including postoperative nausea and vomiting. |
| FDBD01388 | Doripenem |
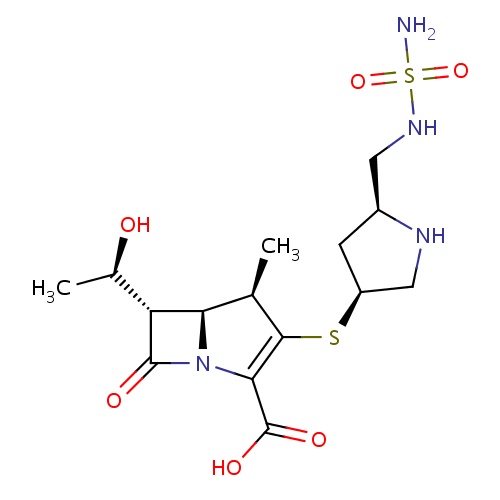
|
Doripenem is indicated in the treatment of complicated intra-abdominal infections and complicated urinary tract infections, including pyelonephritis, caused by designated susceptible bacteria. |
4 ,
1
| FRAGNAME | PDBID | SIMILIRITY | XSCORE | SMILE | HAC |
|---|---|---|---|---|---|
| 3fh5_ligand_1_4.mol2 | 3fh5 | 1 | -6.56 | C[C@H]1CCC[NH2+]1 | 6 |
| 3fun_ligand_1_5.mol2 | 3fun | 1 | -6.56 | [C@H]1([NH2+]CCC1)C | 6 |
| 3fui_ligand_1_0.mol2 | 3fui | 1 | -6.51 | C[C@@H]1[NH2+]CCC1 | 6 |
| 3fh7_ligand_1_4.mol2 | 3fh7 | 1 | -6.32 | C[C@@H]1CCC[NH2+]1 | 6 |
| 2g5p_ligand_1_1.mol2 | 2g5p | 1 | -6.07 | C[C@H]1CCC[NH2+]1 | 6 |
| 2ghg_ligand_1_0.mol2 | 2ghg | 1 | -6.07 | [C@@H]1(CCC[NH2+]1)C | 6 |
| 2g5t_ligand_1_1.mol2 | 2g5t | 1 | -5.95 | C[C@H]1CCC[NH2+]1 | 6 |
| 2c1b_ligand_1_3.mol2 | 2c1b | 1 | -5.94 | C1C[NH2+][C@@H](C1)C | 6 |
333 ,
34

