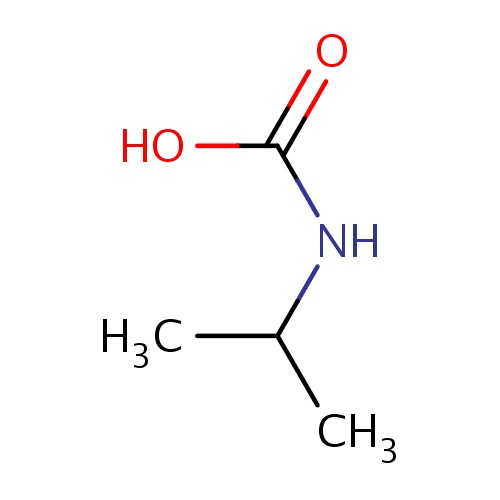
Common name
isopropylcarbamic acid
IUPAC name
isopropylcarbamic acid
SMILES
O=C(O)NC(C)C
Common name
isopropylcarbamic acid
IUPAC name
isopropylcarbamic acid
SMILES
O=C(O)NC(C)C
INCHI
InChI=1S/C4H9NO2/c1-3(2)5-4(6)7/h3,5H,1-2H3,(H,6,7)
FORMULA
C4H9NO2

Common name
isopropylcarbamic acid
IUPAC name
isopropylcarbamic acid
Molecular weight
103.120
clogP
-0.665
clogS
-0.055
Frequency
0.0010
HBond Acceptor
2
HBond Donor
2
Total PolarSurface Area
49.33
Number of Rings
0
Rotatable Bond
1
| Drug ID | Common name | Structure CAS | Compound class | Therapeutic area |
|---|---|---|---|---|
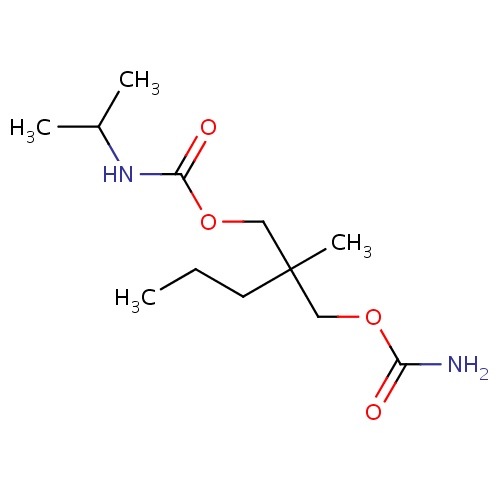
| FDBD00275 | Carisoprodol |
|
Muscle Relaxants, Central; Neuromuscular Agents; Muscle Relaxants, Skeletal; Musculo-Skeletal System; Muscle Relaxants, Centrally Acting Agents; Muscle Relaxants; Carbamic Acid Esters; Cytochrome P-450 CYP2C19 Inducers; | For the relief of discomfort associated with acute, painful, musculoskeletal conditions. |
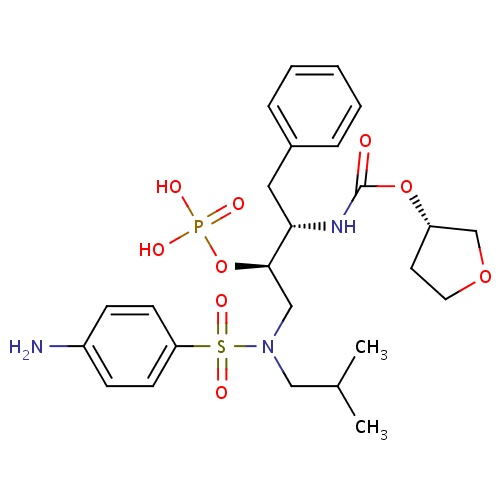
| FDBD01127 | Fosamprenavir |
|
Anti-HIV Agents; Protease Inhibitors; HIV Protease Inhibitors; Prodrugs; Antiinfectives for Systemic Use; Direct Acting Antivirals; Antivirals for Systemic Use; CYP3A4 Inhibitors; | Indicated in combination with other antiretroviral agents for the treatment of human immunodeficiency virus (HIV-1) infection, as well as postexposure prophylaxis of HIV infection in individuals who have had occupational or nonoccupational exposure to potentially infectious body fluids of a person known to be infected with HIV when that exposure represents a substantial risk for HIV transmission. The use of fosamprenavir is pending revision due to a potential association between the drug and myocardial infarction and dyslipidemia in HIV infected adults. |
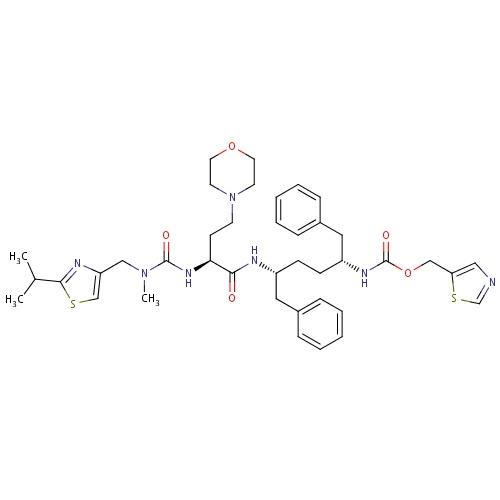
| FDBD01652 | Cobicistat |
|
Anti-HIV Agents; Antiinfectives for Systemic Use; Direct Acting Antivirals; Antivirals for Systemic Use; Cytochrome P-450 CYP3A Inhibitors; CYP2D6 Inducers; CYP2D6 Inducers (strong); | Cobicistat is a CYP3A inhibitor indicated to increase systemic exposure of atazanavir or darunavir (once daily dosing regimen) in combination with other antiretroviral agents in the treatment of HIV-1 infection. It is not interchangeable with ritonavir to increase systemic exposure of darunavir 600 mg twice daily, fosamprenavir, saquinavir, or tipranavir due to lack of exposure data. The use of cobicistat is not recommended with darunavir 600 mg twice daily, fosamprenavir, saquinavir or tipranavir. Complex or unknown mechanisms of drug interactions preclude extrapolation of ritonavir drug interactions to certain cobicistat interactions. Cobicistat and ritonavir when administered with either atazanavir or darunavir may result in different drug interactions when used with concomitant medications. |
3 ,
1
| FRAGNAME | PDBID | SIMILIRITY | XSCORE | SMILE | HAC |
|---|---|---|---|---|---|
| 1m0b_ligand_3_136.mol2 | 1m0b | 1 | -5.83 | CC(NC(=O)O)C | 7 |
| 1lzq_ligand_3_136.mol2 | 1lzq | 1 | -5.82 | CC(NC(=O)O)C | 7 |
| 2pjb_ligand_3_144.mol2 | 2pjb | 1 | -5.27 | CC(C)NC(=O)O | 7 |
| 4gtr_ligand_1_1.mol2 | 4gtr | 0.933333 | -5.86 | N(C(=O)O)CC | 6 |
| 4gtr_ligand_1_0.mol2 | 4gtr | 0.933333 | -5.75 | N(C(=O)O)CC | 6 |
| 4tmn_ligand_2_50.mol2 | 4tmn | 0.933333 | -5.71 | C(=O)(O)NCC | 6 |
241 ,
25

