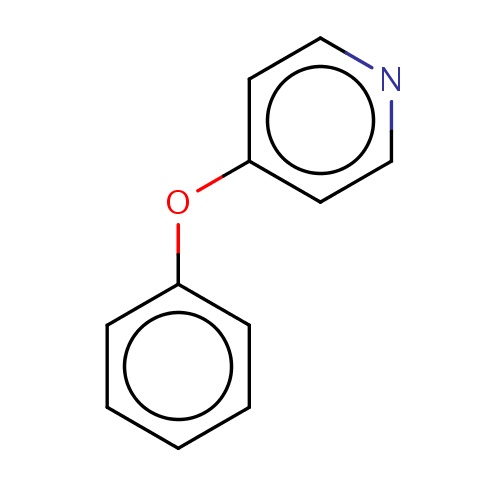
Common name
4-phenoxypyridine
IUPAC name
4-phenoxypyridine
SMILES
O(c1ccccc1)c2ccncc2
Common name
4-phenoxypyridine
IUPAC name
4-phenoxypyridine
SMILES
O(c1ccccc1)c2ccncc2
INCHI
InChI=1S/C11H9NO/c1-2-4-10(5-3-1)13-11-6-8-12-9-7-11/h1-9H
FORMULA
C11H9NO

Common name
4-phenoxypyridine
IUPAC name
4-phenoxypyridine
Molecular weight
171.195
clogP
2.562
clogS
-2.914
Frequency
0.0003
HBond Acceptor
2
HBond Donor
0
Total PolarSurface Area
22.12
Number of Rings
2
Rotatable Bond
2
| Drug ID | Common name | Structure CAS | Compound class | Therapeutic area |
|---|---|---|---|---|
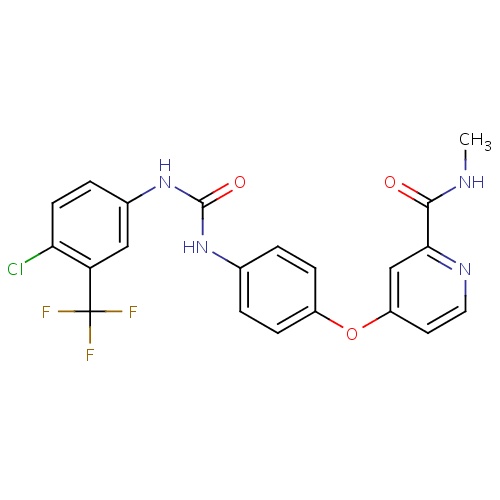
| FDBD00278 | Sorafenib |
|
Antineoplastic Agents; Immunosuppressive Agents; Protein Kinase Inhibitors; Antineoplastic and Immunomodulating Agents; Cytochrome P-450 CYP1A2 Inhibitors; Cytochrome P-450 CYP2C9 Inhibitors; Cytochrome P-450 CYP1A2 Inducers; Cytochrome P-450 CYP2C8 Inhibitors; Cytochrome P-450 CYP2C9 Inducers; Cytochrome P-450 CYP2C19 Inducers; Cytochrome P-450 CYP2C8 Inducers; Cytochrome P-450 CYP2B6 Inducers; Cytochrome P-450 CYP2B6 Inhibitors; CYP2B6 Inhibitors (strong); CYP2D6 Inducers; CYP2D6 Inducers (strong); CYP3A4 Inhibitors; Combined Inhibitors of CYP3A4 and P-glycoprotein; | Sorafenib is indicated for the treatment of unresectable hepatocellular carcinoma and advanced renal cell carcinoma. |
1 ,
1
| FRAGNAME | PDBID | SIMILIRITY | XSCORE | SMILE | HAC |
|---|---|---|---|---|---|
| 5ar7_ligand_2_5.mol2 | 5ar7 | 1 | -7.67 | O(c1ccccc1)c1ccncc1 | 13 |
| 1uwh_ligand_2_9.mol2 | 1uwh | 1 | -7.56 | O(c1ccccc1)c1ccncc1 | 13 |
| 2hzn_ligand_2_6.mol2 | 2hzn | 1 | -7.49 | c1(ccncc1)Oc1ccccc1 | 13 |
| 4eyj_ligand_2_5.mol2 | 4eyj | 1 | -7.43 | c1(ccncc1)Oc1ccccc1 | 13 |
| 3heg_ligand_2_9.mol2 | 3heg | 1 | -7.36 | c1cnccc1Oc1ccccc1 | 13 |
| 3gcs_ligand_2_9.mol2 | 3gcs | 1 | -7.16 | c1ccc(cc1)Oc1ccncc1 | 13 |
| 4eym_ligand_2_9.mol2 | 4eym | 1 | -7.10 | c1(ccncc1)Oc1ccccc1 | 13 |
| 3cd8_ligand_2_5.mol2 | 3cd8 | 0.790698 | -7.18 | c1(c2c(cccc2)ncc1)OC | 12 |
112 ,
12

