
Common name
8-chloro-1-methyl-4H-[1,2,4]triazolo[4,3-a][1,4]benzodiazepine
IUPAC name
8-chloro-1-methyl-4H-[1,2,4]triazolo[4,3-a][1,4]benzodiazepine
SMILES
Clc1cc2c(cc1)-n3c(nnc3C)CN=C2
Common name
8-chloro-1-methyl-4H-[1,2,4]triazolo[4,3-a][1,4]benzodiazepine
IUPAC name
8-chloro-1-methyl-4H-[1,2,4]triazolo[4,3-a][1,4]benzodiazepine
SMILES
Clc1cc2c(cc1)-n3c(nnc3C)CN=C2
INCHI
InChI=1S/C11H9ClN4/c1-7-14-15-11-6-13-5-8-4-9(12)2-3-10(8)16(7)11/h2-5H,6H2,1H3
FORMULA
C11H9ClN4

Common name
8-chloro-1-methyl-4H-[1,2,4]triazolo[4,3-a][1,4]benzodiazepine
IUPAC name
8-chloro-1-methyl-4H-[1,2,4]triazolo[4,3-a][1,4]benzodiazepine
Molecular weight
233.677
clogP
1.545
clogS
-3.480
Frequency
0.0010
HBond Acceptor
2
HBond Donor
1
Total PolarSurface Area
44.92
Number of Rings
3
Rotatable Bond
0
| Drug ID | Common name | Structure CAS | Compound class | Therapeutic area |
|---|---|---|---|---|
| FDBD00284 | Alprazolam |
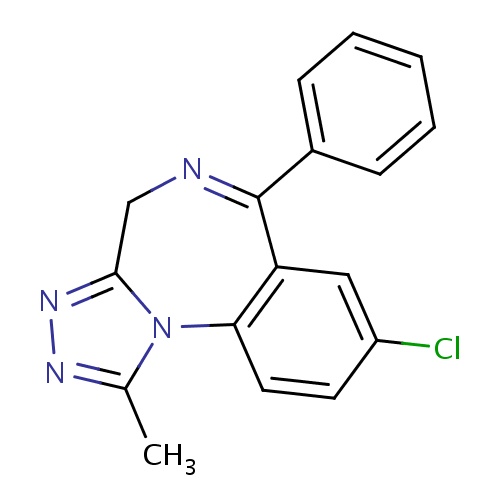
|
Anti-Anxiety Agents; Hypnotics and Sedatives; GABA Modulators; Benzodiazepines; Nervous System; Benzodiazepine Derivatives; Anxiolytics; Psycholeptics; Cytochrome P-450 CYP2C9 Inhibitors; Cytochrome P-450 CYP2C9 Inducers; CYP3A4 Inhibitors; | For the management of anxiety disorder or the short-term relief of symptoms of anxiety and for the treatment of panic disorder, with or without agoraphobia. |
| FDBD00413 | Adinazolam |
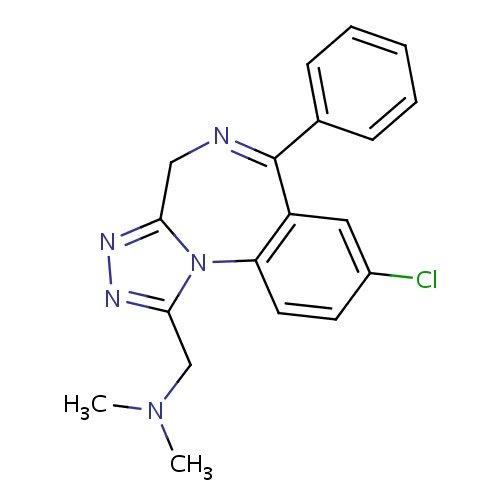
|
Antidepressive Agents; Nervous System; Benzodiazepine Derivatives; Anxiolytics; Psycholeptics; Cytochrome P-450 CYP2C19 Inducers; CYP3A4 Inhibitors; | For the treatment of anxiety and status epilepticus. |
| FDBD00755 | Triazolam |
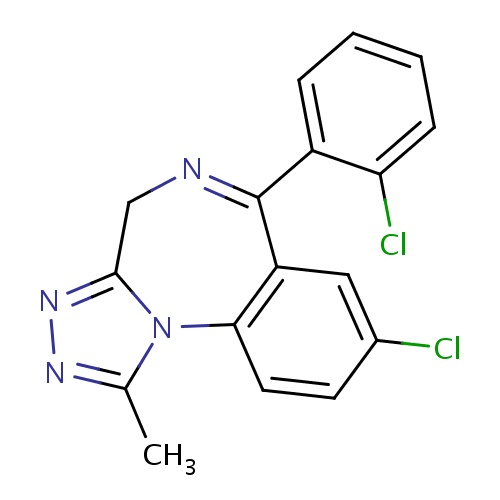
|
Anti-Anxiety Agents; Hypnotics and Sedatives; Adjuvants, Anesthesia; GABA Modulators; Benzodiazepines; Nervous System; Benzodiazepine Derivatives; Psycholeptics; Cytochrome P-450 CYP2C9 Inhibitors; Cytochrome P-450 CYP2C8 Inhibitors; Cytochrome P-450 CYP2C9 Inducers; Cytochrome P-450 CYP2C8 Inducers; CYP3A4 Inhibitors; | For the short-term treatment of insomnia. |
3 ,
1
| FRAGNAME | PDBID | SIMILIRITY | XSCORE | SMILE | HAC |
|---|---|---|---|---|---|
| 2yel_ligand_frag_2.mol2 | 2yel | 0.93038 | -7.32 | c1ccc2c(c1)C=NCc1[nH]nc([n+]21)C | 15 |
| 2yek_ligand_frag_1.mol2 | 2yek | 0.93038 | -7.24 | C1=NCc2[n+](c3c1cccc3)c([nH]n2)C | 15 |
| 2yem_ligand_frag_2.mol2 | 2yem | 0.93038 | -7.24 | c1ccc2c(c1)C=NCc1[nH]nc([n+]21)C | 15 |
| 2ydw_ligand_frag_2.mol2 | 2ydw | 0.93038 | -7.15 | c1ccc2c(c1)C=NCc1[nH]nc([n+]21)C | 15 |
| 4qew_ligand_frag_3.mol2 | 4qew | 0.93038 | -7.14 | C1N=Cc2c([n+]3c1[nH]nc3C)cccc2 | 15 |
| 4qev_ligand_frag_2.mol2 | 4qev | 0.93038 | -7.10 | C1N=Cc2c([n+]3c1[nH]nc3C)cccc2 | 15 |
| 2yek_ligand_1_2.mol2 | 2yek | 0.859649 | -7.61 | C[C@@H]1N=Cc2c([n+]3c1n[nH]c3C)cccc2 | 16 |
100 ,
11

