
Common name
(1R,3S,4S)-4-[(Z)-pent-2-enyl]cyclopentane-1,3-diol
IUPAC name
(1R,3S,4S)-4-[(Z)-pent-2-enyl]cyclopentane-1,3-diol
SMILES
C(C=CCC1CC(CC1O)O)C
Common name
(1R,3S,4S)-4-[(Z)-pent-2-enyl]cyclopentane-1,3-diol
IUPAC name
(1R,3S,4S)-4-[(Z)-pent-2-enyl]cyclopentane-1,3-diol
SMILES
C(C=CCC1CC(CC1O)O)C
INCHI
InChI=1S/C10H18O2/c1-2-3-4-5-8-6-9(11)7-10(8)12/h3-4,8-12H,2,5-7H2,1H3/b4-3-/t8-,9+,10-/m0/s1
FORMULA
C10H18O2

Common name
(1R,3S,4S)-4-[(Z)-pent-2-enyl]cyclopentane-1,3-diol
IUPAC name
(1R,3S,4S)-4-[(Z)-pent-2-enyl]cyclopentane-1,3-diol
Molecular weight
170.249
clogP
1.501
clogS
-0.707
Frequency
0.0003
HBond Acceptor
2
HBond Donor
2
Total PolarSurface Area
40.46
Number of Rings
1
Rotatable Bond
3
| Drug ID | Common name | Structure CAS | Compound class | Therapeutic area |
|---|---|---|---|---|
| FDBD00306 | Carboprost Tromethamine |
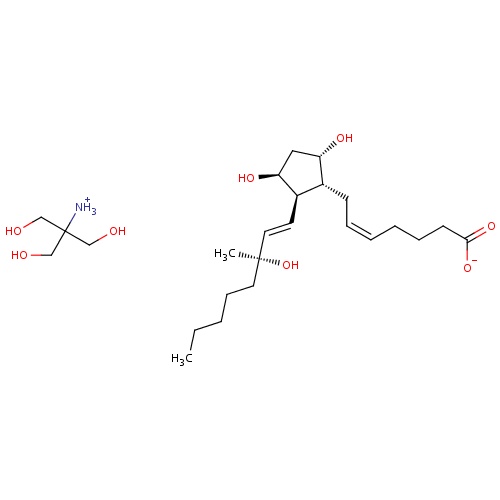
|
Oxytocics; Abortifacient Agents, Nonsteroidal; Prostaglandins; Genito Urinary System and Sex Hormones; Uterotonics; Tromethamine; | For aborting pregnancy between the 13th and 20th weeks of gestation as calculated from the first day of the last normal menstrual period and in the following conditions related to second trimester abortion: 1. Failure of expulsion of the fetus during the course of treatment by another method; 2. Premature rupture of membranes in intrauterine methods with loss of drug and insufficient or absent uterine activity; 3. Requirement of a repeat intrauterine instillation of drug for expulsion of the fetus; 4. Inadvertent or spontaneous rupture of membranes in the presence of a previable fetus and absence of adequate activity for expulsion. Also for the treatment of postpartum hemorrhage due to uterine atony which has not responded to conventional methods of management. |
1 ,
1
| FRAGNAME | PDBID | SIMILIRITY | XSCORE | SMILE | HAC |
|---|---|---|---|---|---|
| 1o9e_ligand_2_1.mol2 | 1o9e | 0.823529 | -8.00 | C[C@@H]1C2=C[C@@]3([C@@H]([C@@H](C[C@@H]3O)CC)C[C@@H]([C@@H]([C@@H]2CC1)C)O)C | 21 |
| 1o9e_ligand_1_2.mol2 | 1o9e | 0.823529 | -7.76 | C[C@@H]1C2=C[C@@]3([C@@H](CC[C@@H]3O)C[C@@H]([C@@H]([C@@H]2CC1)C)O)C | 19 |
| 1o9e_ligand_1_0.mol2 | 1o9e | 0.823529 | -7.69 | [C@@H]12[C@@H](C[C@@H]([C@@]1(C=C1CCC[C@H]1[C@H]([C@H](C2)O)C)C)O)CC | 20 |
| 1o9e_ligand_frag_0.mol2 | 1o9e | 0.823529 | -7.45 | [C@@H]12CC[C@@H]([C@@]1(C=C1CCC[C@H]1[C@H]([C@H](C2)O)C)C)O | 18 |
| 3e6y_ligand_1_6.mol2 | 3e6y | 0.8 | -7.86 | C(C)(C)[C@H]1[C@@H]2C[C@@H]([C@@H]([C@H]3C(=C[C@]2(CC1)C)[C@@H](CC3)O)C)O | 21 |
| 3e6y_ligand_1_7.mol2 | 3e6y | 0.8 | -7.86 | C[C@@]1(C2=C[C@@]3([C@@H](C[C@@H]([C@@H]([C@@H]2CC1)C)O)CCC3)C)O | 19 |
| 3e6y_ligand_frag_6.mol2 | 3e6y | 0.8 | -7.50 | C1[C@H]2CCC[C@@]2(C=C2[C@@H](CC[C@H]2[C@H]([C@H]1O)C)O)C | 18 |
| 3ihz_ligand_1_4.mol2 | 3ihz | 0.741935 | -6.66 | [C@@H]1(CC[C@@H](CC1)O)/C=C\C | 10 |
| 1fkf_ligand_1_3.mol2 | 1fkf | 0.741935 | -6.43 | [C@@H]1(CC[C@@H](CC1)O)/C=C\C | 10 |
100 ,
11

