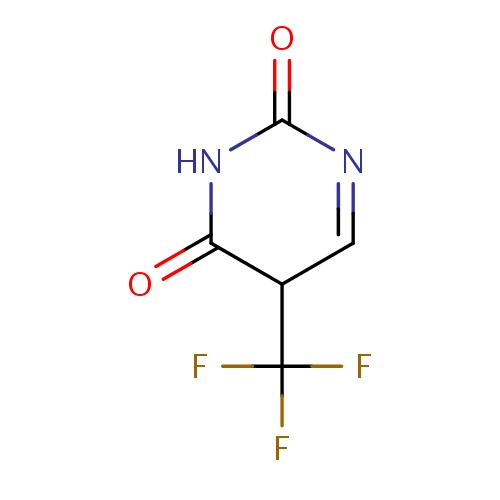
Common name
(5S)-5-(trifluoromethyl)-5H-pyrimidine-2,4-dione
IUPAC name
(5S)-5-(trifluoromethyl)-5H-pyrimidine-2,4-dione
SMILES
O=C1N=CC(C(=O)N1)C(F)(F)F
Common name
(5S)-5-(trifluoromethyl)-5H-pyrimidine-2,4-dione
IUPAC name
(5S)-5-(trifluoromethyl)-5H-pyrimidine-2,4-dione
SMILES
O=C1N=CC(C(=O)N1)C(F)(F)F
INCHI
InChI=1S/C5H3F3N2O2/c6-5(7,8)2-1-9-4(12)10-3(2)11/h1-2H,(H,10,11,12)
FORMULA
C5H3F3N2O2

Common name
(5S)-5-(trifluoromethyl)-5H-pyrimidine-2,4-dione
IUPAC name
(5S)-5-(trifluoromethyl)-5H-pyrimidine-2,4-dione
Molecular weight
180.085
clogP
1.397
clogS
-1.288
Frequency
0.0003
HBond Acceptor
2
HBond Donor
1
Total PolarSurface Area
58.53
Number of Rings
1
Rotatable Bond
1
| Drug ID | Common name | Structure CAS | Compound class | Therapeutic area |
|---|---|---|---|---|
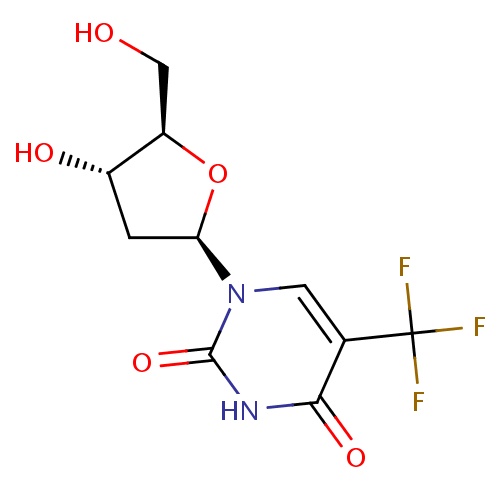
| FDBD00309 | Trifluridine |
|
Antineoplastic Agents; Antiviral Agents; Antimetabolites; Antineoplastic and Immunomodulating Agents; Ophthalmologicals; Sensory Organs; Antiinfectives; Pyrimidine Analogues; | Ophthalmic solution for the treatment of primay keratoconjunctivitis and recurrent epithelial keratitis due to herpes simplex virus, types 1 and 2. |
1 ,
1
| FRAGNAME | PDBID | SIMILIRITY | XSCORE | SMILE | HAC |
|---|---|---|---|---|---|
| 4tmk_ligand_frag_0.mol2 | 4tmk | 0.84 | -6.99 | N1=C[C@H](C(=O)NC1=O)C | 9 |
| 4uxh_ligand_frag_0.mol2 | 4uxh | 0.84 | -6.96 | N1=C[C@H](C(=O)NC1=O)C | 9 |
| 5tmp_ligand_frag_0.mol2 | 5tmp | 0.84 | -6.95 | N1=C[C@H](C(=O)NC1=O)C | 9 |
| 1mrn_ligand_frag_0.mol2 | 1mrn | 0.84 | -6.83 | N1=C[C@@H](C(=O)NC1=O)C | 9 |
| 4uxj_ligand_frag_8.mol2 | 4uxj | 0.84 | -6.82 | N1=C[C@H](C(=O)NC1=O)C | 9 |
| 1w2g_ligand_frag_2.mol2 | 1w2g | 0.84 | -6.80 | N1=C[C@H](C(=O)NC1=O)C | 9 |
| 2j87_ligand_frag_8.mol2 | 2j87 | 0.84 | -6.77 | N1=C[C@@H](C(=O)NC1=O)C | 9 |
| 4hdc_ligand_frag_7.mol2 | 4hdc | 0.84 | -6.73 | N1=C[C@H](C(=O)NC1=O)C | 9 |
102 ,
11

