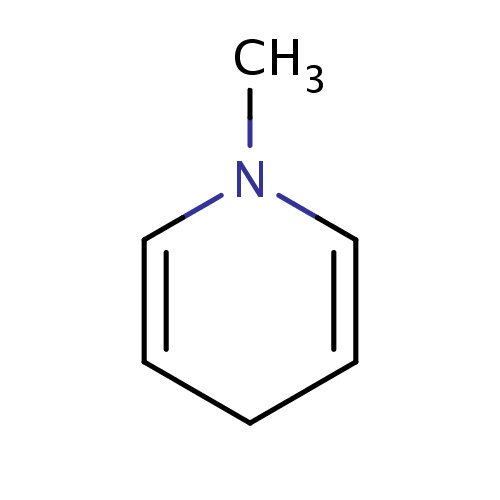
Common name
1-methyl-4H-pyridine
IUPAC name
1-methyl-4H-pyridine
SMILES
N1(C=CCC=C1)C
Common name
1-methyl-4H-pyridine
IUPAC name
1-methyl-4H-pyridine
SMILES
N1(C=CCC=C1)C
INCHI
InChI=1S/C6H9N/c1-7-5-3-2-4-6-7/h3-6H,2H2,1H3
FORMULA
C6H9N

Common name
1-methyl-4H-pyridine
IUPAC name
1-methyl-4H-pyridine
Molecular weight
95.142
clogP
0.670
clogS
0.033
Frequency
0.0010
HBond Acceptor
1
HBond Donor
0
Total PolarSurface Area
3.24
Number of Rings
1
Rotatable Bond
0
| Drug ID | Common name | Structure CAS | Compound class | Therapeutic area |
|---|---|---|---|---|
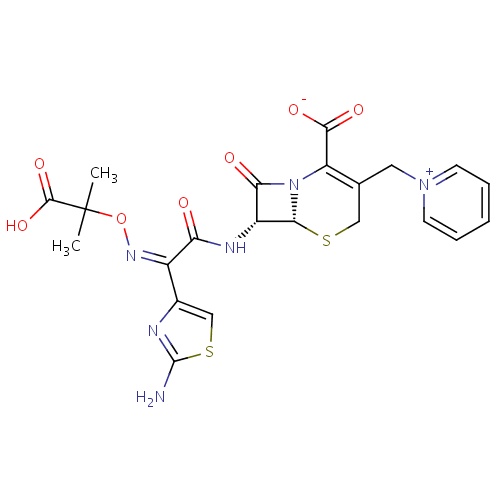
| FDBD00315 | Ceftazidime |
|
Anti-Bacterial Agents; Cephalosporins; Antibacterials for Systemic Use; Antiinfectives for Systemic Use; Third-Generation Cephalosporins; | For the treatment of patients with infections caused by susceptible strains of organisms in the following diseases: lower respiratory tract infections,skin and skin structure infections, urinary tract infections, bacterial septicemia, bone and joint infections, gynecologic infections, intra abdominal infections (including peritonitis), and central nervous system infections (including meningitis). |
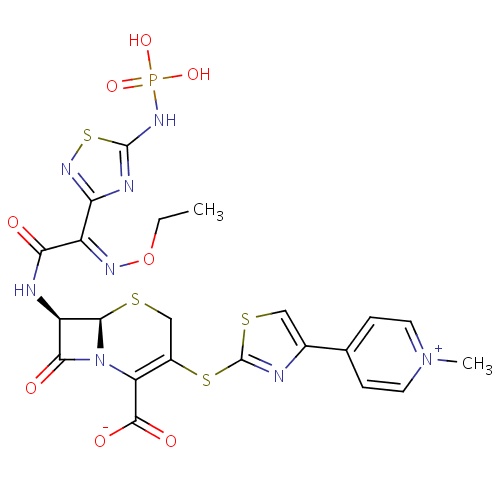
| FDBD01420 | Ceftaroline fosamil |
|
Cephalosporins; Antibiotics; Antibacterials for Systemic Use; Antiinfectives for Systemic Use; | Ceftaroline fosamil is indicated for the treatment of patients with the following infections caused by susceptible isolates of the designated microorganisms. |
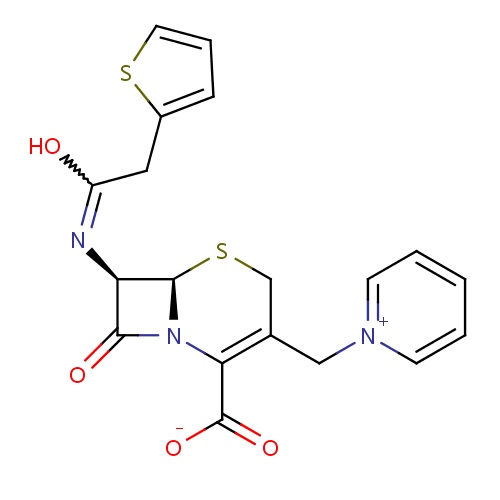
| FDBD01622 | Cephaloridine |
|
Anti-Bacterial Agents; |
3 ,
1
| FRAGNAME | PDBID | SIMILIRITY | XSCORE | SMILE | HAC |
|---|---|---|---|---|---|
| 5bwc_ligand_1_7.mol2 | 5bwc | 1 | -6.01 | CN1C=CCC=C1 | 7 |
| 5ael_ligand_1_4.mol2 | 5ael | 1 | -5.99 | CN1C=CCC=C1 | 7 |
| 4cg8_ligand_1_8.mol2 | 4cg8 | 1 | -5.93 | CN1C=CCC=C1 | 7 |
| 5bwc_ligand_2_33.mol2 | 5bwc | 0.902439 | -6.29 | C(C)N1C=CCC=C1 | 8 |
| 5ael_ligand_2_22.mol2 | 5ael | 0.902439 | -6.23 | C(N1C=CCC=C1)C | 8 |
| 5bwc_ligand_3_80.mol2 | 5bwc | 0.840909 | -6.49 | C(CC)N1C=CCC=C1 | 9 |
| 4o70_ligand_frag_3.mol2 | 4o70 | 0.837209 | -5.56 | C1C=C[N+](C=C1)O | 7 |
| 5bwc_ligand_4_121.mol2 | 5bwc | 0.787234 | -6.69 | C(CCC)N1C=CCC=C1 | 10 |
| 4o70_ligand_1_2.mol2 | 4o70 | 0.75 | -5.71 | CC1=C[N+](C=CC1)O | 8 |
| 5ael_ligand_2_18.mol2 | 5ael | 0.72549 | -6.25 | C#CC1=CN(C)C=CC1 | 9 |
111 ,
12

