
Common name
1,2,3-trimethoxy-5-methyl-benzene
IUPAC name
1,2,3-trimethoxy-5-methyl-benzene
SMILES
Cc1cc(c(c(c1)OC)OC)OC
Common name
1,2,3-trimethoxy-5-methyl-benzene
IUPAC name
1,2,3-trimethoxy-5-methyl-benzene
SMILES
Cc1cc(c(c(c1)OC)OC)OC
INCHI
InChI=1S/C10H14O3/c1-7-5-8(11-2)10(13-4)9(6-7)12-3/h5-6H,1-4H3
FORMULA
C10H14O3

Common name
1,2,3-trimethoxy-5-methyl-benzene
IUPAC name
1,2,3-trimethoxy-5-methyl-benzene
Molecular weight
182.216
clogP
2.313
clogS
-2.865
Frequency
0.0010
HBond Acceptor
3
HBond Donor
0
Total PolarSurface Area
27.69
Number of Rings
1
Rotatable Bond
3
| Drug ID | Common name | Structure CAS | Compound class | Therapeutic area |
|---|---|---|---|---|
| FDBD00316 | Trimethoprim |
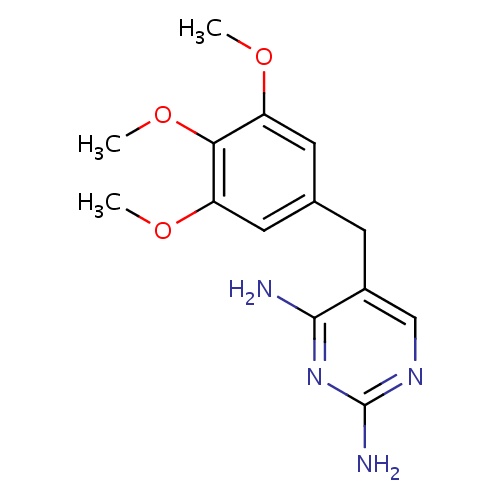
|
Antimalarials; Folic Acid Antagonists; Anti-Infective Agents; Anti-Infective Agents, Urinary; Antibacterials for Systemic Use; Antiinfectives for Systemic Use; Antibacterials for Intramammary Use; Sulfonamides and Trimethoprim; Sulfonamides and Trimethoprim Incl. Derivatives; Trimethoprim and Derivatives; Sulfonamides and Trimethoprim for Intramammary Use; Cytochrome P-450 CYP2C9 Inhibitors; Cytochrome P-450 CYP2C8 Inhibitors; Cytochrome P-450 CYP2C9 Inducers; Cytochrome P-450 CYP2C8 Inducers; CYP3A4 Inhibitors; | For the treatment of urinary tract infections, uncomplicated pyelonephritis (with sulfamethoxazole) and mild acute prostatitis. May be used as pericoital (with sulfamethoxazole) or continuous prophylaxis in females with recurrent cystitis. May be used as an alternative to treat asymptomatic bacteriuria during pregnancy (only before the last 6 weeks of pregnancy). Other uses include: alternative agent in respiratory tract infections (otitis, sinusitus, bronchitis and pneumonia), treatment of Pneumocystis jirovecii pneumonia (acute or prophylaxis), Nocardia infections, and traveller's diarrhea. |
| FDBD03005 | metrafenone |

|
Fungicide | Fungicide |
| FDBD03006 | pyriofenone |

|
Fungicide | Fungicide |
3 ,
1
| FRAGNAME | PDBID | SIMILIRITY | XSCORE | SMILE | HAC |
|---|---|---|---|---|---|
| 1uy6_ligand_4_0.mol2 | 1uy6 | 1 | -6.63 | O(C)c1cc(cc(c1OC)OC)C | 13 |
| 1uym_ligand_4_0.mol2 | 1uym | 1 | -6.58 | Cc1cc(OC)c(c(c1)OC)OC | 13 |
| 3fl9_ligand_4_4.mol2 | 3fl9 | 1 | -6.31 | O(C)c1c(cc(cc1OC)C)OC | 13 |
| 3n0h_ligand_4_4.mol2 | 3n0h | 1 | -6.30 | O(C)c1cc(cc(c1OC)OC)C | 13 |
| 2w9h_ligand_4_4.mol2 | 2w9h | 1 | -6.21 | c1(cc(c(c(c1)OC)OC)OC)C | 13 |
| 4km2_ligand_4_4.mol2 | 4km2 | 1 | -6.09 | O(C)c1c(cc(cc1OC)C)OC | 13 |
| 1bl4_ligand_4_650.mol2 | 1bl4 | 1 | -5.60 | Cc1cc(OC)c(c(c1)OC)OC | 13 |
| 4tw7_ligand_4_2379.mol2 | 4tw7 | 1 | -5.57 | c1(cc(c(c(c1)OC)OC)OC)C | 13 |
| 4tw6_ligand_4_0.mol2 | 4tw6 | 1 | -5.51 | Cc1cc(c(OC)c(OC)c1)OC | 13 |
103 ,
11

