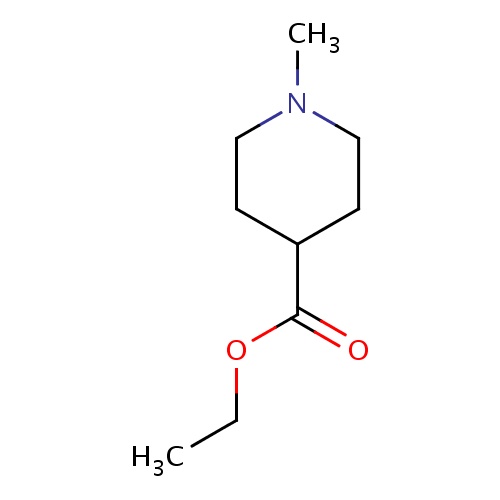
Common name
ethyl 1-methylpiperidine-4-carboxylate
IUPAC name
ethyl 1-methylpiperidine-4-carboxylate
SMILES
N1(CCC(CC1)C(=O)OCC)C
Common name
ethyl 1-methylpiperidine-4-carboxylate
IUPAC name
ethyl 1-methylpiperidine-4-carboxylate
SMILES
N1(CCC(CC1)C(=O)OCC)C
INCHI
InChI=1S/C9H17NO2/c1-3-12-9(11)8-4-6-10(2)7-5-8/h8H,3-7H2,1-2H3
FORMULA
C9H17NO2

Common name
ethyl 1-methylpiperidine-4-carboxylate
IUPAC name
ethyl 1-methylpiperidine-4-carboxylate
Molecular weight
171.237
clogP
1.117
clogS
-1.237
Frequency
0.0010
HBond Acceptor
3
HBond Donor
0
Total PolarSurface Area
29.54
Number of Rings
1
Rotatable Bond
3
| Drug ID | Common name | Structure CAS | Compound class | Therapeutic area |
|---|---|---|---|---|
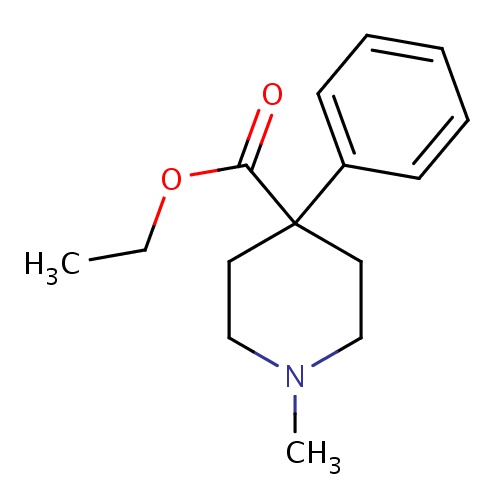
| FDBD00329 | Pethidine |
|
Analgesics; Analgesics, Opioid; Narcotics; Adjuvants, Anesthesia; Adjuvants; Nervous System; Opioids; Phenylpiperidine Derivatives; Cytochrome P-450 CYP2C19 Inducers; Cytochrome P-450 CYP2B6 Inducers; Cytochrome P-450 CYP2B6 Inhibitors; CYP2B6 Inhibitors (strong); CYP2D6 Inducers; CYP2D6 Inducers (strong); CYP3A4 Inhibitors; | Used to control moderate to severe pain. |
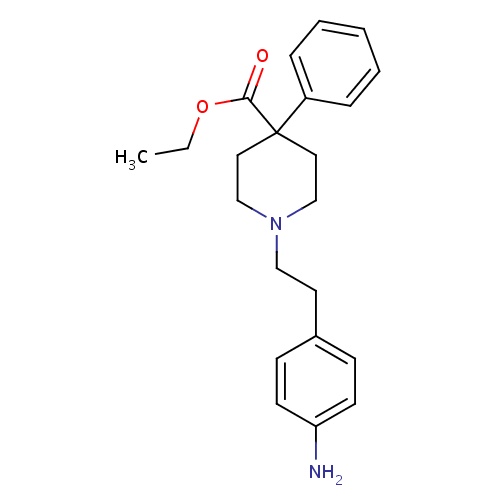
| FDBD00770 | Anileridine |
|
Anesthetics; Nervous System; Anesthetics, General; Opioid Anesthetics; | For treatment and management of pain (systemic) and for use as an anesthesia adjunct. |
| FDBD00931 | Diphenoxylate |

|
Analgesics, Opioid; Antidiarrheals; Antiperistaltic Agents; Alimentary Tract and Metabolism; Antidiarrheals, Intestinal Anti-Inflammatory/antiinfective Agents; Antipropulsives; | For as adjunctive therapy in the management of diarrhea. |
3 ,
1
| FRAGNAME | PDBID | SIMILIRITY | XSCORE | SMILE | HAC |
|---|---|---|---|---|---|
| 1k1j_ligand_1_3.mol2 | 1k1j | 0.804348 | -5.85 | C(=O)(N1CCC(CC1)C(=O)OC)C | 13 |
| 1k1j_ligand_frag_3.mol2 | 1k1j | 0.804348 | -5.66 | C(=O)N1CCC(CC1)C(=O)OC | 12 |
| 3fuk_ligand_2_0.mol2 | 3fuk | 0.756098 | -6.90 | CC[N@H+]1CC[C@@H](C(=O)O)CC1 | 11 |
| 3fuk_ligand_1_0.mol2 | 3fuk | 0.756098 | -6.57 | C[N@H+]1CC[C@@H](C(=O)O)CC1 | 10 |
| 3fuk_ligand_frag_0.mol2 | 3fuk | 0.731707 | -6.44 | O=C(O)C1CC[NH2+]CC1 | 9 |
| 2xke_ligand_frag_0.mol2 | 2xke | 0.731707 | -6.20 | C1CC(CC[NH2+]1)C(=O)O | 9 |
| 2xkf_ligand_frag_3.mol2 | 2xkf | 0.731707 | -6.09 | [NH2+]1CCC(CC1)C(=O)O | 9 |
| 2g8r_ligand_frag_3.mol2 | 2g8r | 0.731707 | -5.97 | [NH2+]1CCC(CC1)C(=O)O | 9 |
115 ,
12

