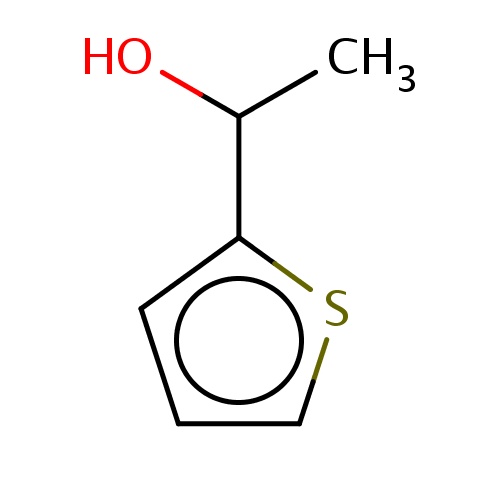
Common name
(1S)-1-(2-thienyl)ethanol
IUPAC name
(1S)-1-(2-thienyl)ethanol
SMILES
C(O)(c1sccc1)C
Common name
(1S)-1-(2-thienyl)ethanol
IUPAC name
(1S)-1-(2-thienyl)ethanol
SMILES
C(O)(c1sccc1)C
INCHI
InChI=1S/C6H8OS/c1-5(7)6-3-2-4-8-6/h2-5,7H,1H3/t5-/m0/s1
FORMULA
C6H8OS

Common name
(1S)-1-(2-thienyl)ethanol
IUPAC name
(1S)-1-(2-thienyl)ethanol
Molecular weight
128.192
clogP
2.504
clogS
-1.033
Frequency
0.0003
HBond Acceptor
2
HBond Donor
1
Total PolarSurface Area
48.47
Number of Rings
1
Rotatable Bond
1
| Drug ID | Common name | Structure CAS | Compound class | Therapeutic area |
|---|---|---|---|---|
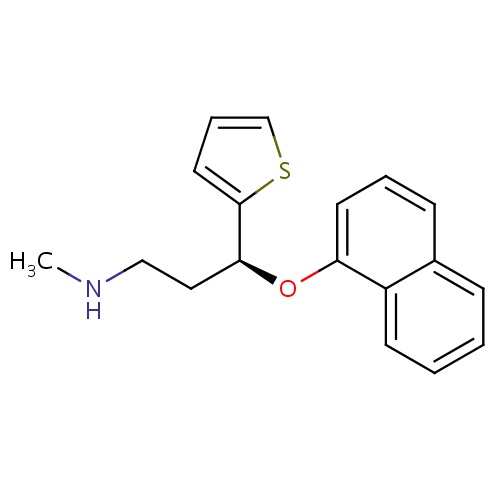
| FDBD00349 | Duloxetine |
|
Dopamine Agents; Analgesics; Antidepressive Agents; Nervous System; Antidepressants; Psychoanaleptics; Cytochrome P-450 CYP1A2 Inhibitors; Cytochrome P-450 CYP1A2 Inducers; Serotonin and Noradrenaline Reuptake Inhibitors; CYP2D6 Inducers; CYP2D6 Inducers (strong); | For the acute and maintenance treatment of major depressive disorder (MDD), as well as acute management of generalized anxiety disorder. Also used for the management of neuropathic pain associated with diabetic peripheral neuropathy, and fibromyalgia. Has been used in the management of moderate to severe stress urinary incontinence (SUI) in women. |
1 ,
1
| FRAGNAME | PDBID | SIMILIRITY | XSCORE | SMILE | HAC |
|---|---|---|---|---|---|
| 4mm6_ligand_3_16.mol2 | 4mm6 | 1 | -6.22 | c1(sccc1)[C@@H](O)C | 8 |
| 4mm6_ligand_4_10.mol2 | 4mm6 | 0.836735 | -6.45 | c1(sccc1)[C@@H](O)CC | 9 |
| 4mm6_ligand_2_12.mol2 | 4mm6 | 0.780488 | -5.81 | c1(sccc1)CO | 7 |
| 4pov_ligand_1_0.mol2 | 4pov | 0.653061 | -6.39 | c1sc(c(c1)C)CO | 8 |
| 4pop_ligand_1_0.mol2 | 4pop | 0.653061 | -6.37 | c1sc(c(c1)C)CO | 8 |
| 2hdq_ligand.mol2 | 2hdq | 0.627451 | -6.43 | O=C(O)c1sccc1 | 9 |
| 4trz_ligand_3_914.mol2 | 4trz | 0.611111 | -6.05 | C(CO)Cc1cccs1 | 9 |
| 1ajq_ligand.mol2 | 1ajq | 0.568966 | -6.84 | s1c(ccc1)CC(=O)O | 10 |
| 4tmr_ligand_frag_0.mol2 | 4tmr | 0.533333 | -5.76 | O=C(OC)c1cccs1 | 9 |
| 2ay8_ligand.mol2 | 2ay8 | 0.532258 | -6.54 | s1c(ccc1)CCCC(=O)O | 12 |
193 ,
20

