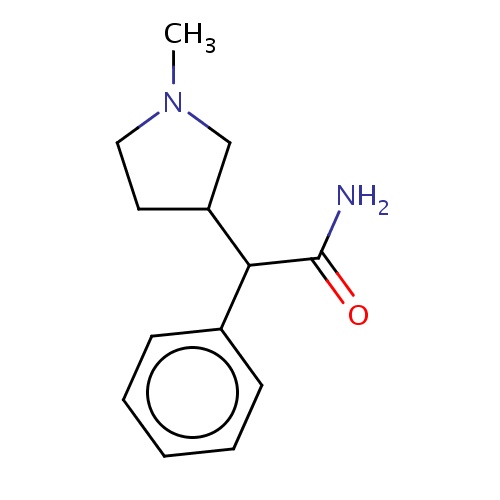
Common name
(2R)-2-[(3S)-1-methylpyrrolidin-3-yl]-2-phenyl-acetamide
IUPAC name
(2R)-2-[(3S)-1-methylpyrrolidin-3-yl]-2-phenyl-acetamide
SMILES
CN1CC(CC1)C(c2ccccc2)C(=O)N
Common name
(2R)-2-[(3S)-1-methylpyrrolidin-3-yl]-2-phenyl-acetamide
IUPAC name
(2R)-2-[(3S)-1-methylpyrrolidin-3-yl]-2-phenyl-acetamide
SMILES
CN1CC(CC1)C(c2ccccc2)C(=O)N
INCHI
InChI=1S/C13H18N2O/c1-15-8-7-11(9-15)12(13(14)16)10-5-3-2-4-6-10/h2-6,11-12H,7-9H2,1H3,(H2,14,16)/t11-,12+/m1/s1
FORMULA
C13H18N2O

Common name
(2R)-2-[(3S)-1-methylpyrrolidin-3-yl]-2-phenyl-acetamide
IUPAC name
(2R)-2-[(3S)-1-methylpyrrolidin-3-yl]-2-phenyl-acetamide
Molecular weight
218.295
clogP
1.550
clogS
-1.888
Frequency
0.0003
HBond Acceptor
2
HBond Donor
2
Total PolarSurface Area
46.33
Number of Rings
2
Rotatable Bond
3
| Drug ID | Common name | Structure CAS | Compound class | Therapeutic area |
|---|---|---|---|---|
| FDBD00369 | Darifenacin |
|
Muscarinic Antagonists; Antispasmodic Agents; Genito Urinary System and Sex Hormones; Drugs for Urinary Frequency and Incontinence; Urological Agents; CYP2D6 Inducers; CYP2D6 Inducers (strong); CYP3A4 Inhibitors; | For the treatment of overactive bladder with symptoms of urge urinary incontinence, urgency and frequency. |
1 ,
1
| FRAGNAME | PDBID | SIMILIRITY | XSCORE | SMILE | HAC |
|---|---|---|---|---|---|
| 1is0_ligand_4_4.mol2 | 1is0 | 0.746269 | -5.98 | c1(ccccc1)[C@@H]1[C@@H](C(=O)NC)[C@@H]1C(=O)NC | 17 |
| 2fv5_ligand_2_13.mol2 | 2fv5 | 0.742424 | -7.42 | c1ccc(cc1)[C@@]1(CCN(CC)C1=O)C | 15 |
| 3cct_ligand_3_13.mol2 | 3cct | 0.72973 | -7.30 | c1cccc(c1)[C@H]1C[N@@H+](C)[C@@H](C(C)C)[C@@H]1C(=O)N | 18 |
| 2fv5_ligand_1_3.mol2 | 2fv5 | 0.712121 | -7.14 | c1ccc(cc1)[C@@]1(CCNC1=O)C | 13 |
| 1is0_ligand_4_488.mol2 | 1is0 | 0.712121 | -6.11 | c1(ccccc1)[C@@H]1[C@@H](C(=O)NCC)C1 | 14 |
| 3cda_ligand_2_33.mol2 | 3cda | 0.694444 | -7.05 | c1(ccccc1)[C@@H]1[C@@H](C(=O)N)[N@@H+](CC1)C(C)C | 17 |
| 3cda_ligand_3_87.mol2 | 3cda | 0.693333 | -7.32 | c1(ccccc1)[C@@H]1[C@@H](C(=O)N)[N@@H+]([C@H](C)C1)C(C)C | 18 |
| 3cda_ligand_2_18.mol2 | 3cda | 0.688312 | -7.19 | c1cc(ccc1[C@@H]1C[N@H+]([C@H](C(=O)N)C1)C(C)C)F | 18 |
| 1mzc_ligand_1_0.mol2 | 1mzc | 0.680556 | -7.09 | c1(ccccc1)[C@H]1C(=O)N(CCCC1)C | 15 |
| 1b5g_ligand_2_9.mol2 | 1b5g | 0.679487 | -6.97 | C1CC[C@@H]2CC[C@](C(=O)N12)([NH3+])Cc1ccccc1 | 18 |
191 ,
20

